FLURAZEPAM HYDROCHLORIDE capsule
Flurazepam Hydrochloride by
Drug Labeling and Warnings
Flurazepam Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by PD-Rx Pharmaceuticals, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use FLURAZEPAM HYDROCHLORIDE CAPSULES safely and effectively. See full prescribing information for FLURAZEPAM HYDROCHLORIDE CAPSULES.
FLURAZEPAM HYDROCHLORIDE capsules, for oral use
Initial U.S. Approval: 1970WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
See full prescribing information for complete boxed warning.
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.1), Drug Interactions (7.1)] .
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
RECENT MAJOR CHANGES
Warnings and Precautions: CNS Depressant Effects and
Next-Day Impairment (5.2) 12/2018INDICATIONS AND USAGE
Flurazepam, a gamma-aminobutyric (GABA A) agonist, is indicated for the treatment of insomnia characterized by difficulty falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. ( 1)
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- 15 mg and 30 mg capsules. ( 3)
WARNINGS AND PRECAUTIONS
- Concomitant use with opioids: May result in profound sedation, respiratory depression, coma and death. Reserve concomitant prescribing of benzodiazepines and opioids for use in patients for whom alternative treatment options are inadequate. ( 5.1)
- CNS depressant effects: Impaired alertness and motor coordination, including risk of falling. Daytime impairment. Caution patients against driving and other activities requiring complete mental alertness. ( 5.2)
- When discontinued, benzodiazepine withdrawal syndrome can occur. ( 5.3)
- The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. ( 5.4)
- Severe anaphylactic/anaphylactoid reactions: Angioedema and anaphylaxis have been reported. Do not rechallenge if such reactions occur. ( 5.5)
- Sleep driving and other complex behaviors while not fully awake: Risk increases with dose and concomitant CNS depressants and alcohol. Immediately evaluate any new onset behavioral changes. ( 5.6)
- Worsening of depression or suicidal thinking may occur: Prescribe the least number of capsules feasible to avoid intentional overdose. ( 5.7)
ADVERSE REACTIONS
Adverse reactions: dizziness, drowsiness, light-headedness, staggering, ataxia, falling. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Mylan at 1-877-446-3679 (1-877-4-INFO-RX) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2018
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
2.2 Dosage in Elderly or Debilitated Patients
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
5.2 CNS Depressant Effects and Next-Day Impairment
5.3 Benzodiazepine Withdrawal Syndrome
5.4 Need to Evaluate for Co-morbid Disorders
5.5 Severe Anaphylactic or Anaphylactoid Reactions
5.6 Abnormal Thinking and Behavior Changes
5.7 Worsening of Depression
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
7 DRUG INTERACTIONS
7.1 Drug Interactions
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Pediatric Use
8.3 Geriatric Use
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
9.2 Abuse and Dependence
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS
Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death [see Warnings and Precautions (5.1), Drug Interactions (7.1)] .
- Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
- Limit dosages and durations to the minimum required.
- Follow patients for signs and symptoms of respiratory depression and sedation.
-
1 INDICATIONS AND USAGE
Flurazepam hydrochloride capsules are indicated for the treatment of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings [see Clinical Studies (14)].
Since insomnia is often transient and intermittent, short-term use is usually sufficient. Prolonged use of hypnotics is usually not indicated and should only be undertaken concomitantly with appropriate evaluation of the patient.
-
2 DOSAGE AND ADMINISTRATION
2.1 Dosage in Adults
Use the lowest dose effective for the patient, as important adverse effects of flurazepam hydrochloride capsules are dose related.
The recommended initial dose is 15 mg for women and either 15 mg or 30 mg for men. The 15 mg dose can be increased to 30 mg if necessary for efficacy.
The recommended initial doses for women and men are different because flurazepam clearance is lower in women [see Pharmacokinetics (12.3)].
2.2 Dosage in Elderly or Debilitated Patients
Elderly or debilitated patients may be especially sensitive to flurazepam. Since the risk of the development of oversedation, dizziness, confusion and/or ataxia increases substantially with larger doses in elderly or debilitated patients, it is recommended that in such patients the dosage be limited to 15 mg. Staggering and falling have also been reported, particularly in geriatric patients [see Warnings and Precautions (5.1)].
-
3 DOSAGE FORMS AND STRENGTHS
Flurazepam Hydrochloride Capsules, USP are available containing either 15 mg or 30 mg of flurazepam hydrochloride, USP.
- The 15 mg capsule is a hard-shell gelatin capsule with a white opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4415 in black ink on both the cap and body.
- The 30 mg capsule is a hard-shell gelatin capsule with a powder blue opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4430 in black ink on both the cap and body.
-
4 CONTRAINDICATIONS
Flurazepam hydrochloride capsules are contraindicated in patients with known hypersensitivity to flurazepam or other benzodiazepines. Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of flurazepam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Patients who develop such reactions should not be rechallenged with flurazepam.
-
5 WARNINGS AND PRECAUTIONS
5.1 Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including flurazepam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of benzodiazepines and opioids for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe flurazepam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. Advise both patients and caregivers about the risks of respiratory depression and sedation when flurazepam is used with opioids [see Drug Interactions (7.1), Patient Counseling Information (17)] .
5.2 CNS Depressant Effects and Next-Day Impairment
Dizziness, drowsiness, light-headedness, staggering, ataxia and falling can occur, particularly in elderly or debilitated persons. Severe sedation, lethargy, disorientation and coma, probably indicative of drug intolerance or overdosage, have been reported.Dizziness, drowsiness, light-headedness, staggering, ataxia and falling can occur, particularly in elderly or debilitated persons. Severe sedation, lethargy, disorientation and coma, probably indicative of drug intolerance or overdosage, have been reported.
Flurazepam is a central nervous system (CNS) depressant and can impair daytime function even when used as prescribed. Prescribers should monitor for excess depressant effects, but impairment can occur in the absence of subjective symptoms, and may not be reliably detected by ordinary clinical exam (i.e., less than formal psychomotor testing). While pharmacodynamic tolerance or adaptation to some adverse depressant effects of flurazepam may develop, patients using flurazepam should be cautioned against driving or engaging in other hazardous activities or activities requiring complete mental alertness.Flurazepam is a central nervous system (CNS) depressant and can impair daytime function even when used as prescribed. Prescribers should monitor for excess depressant effects, but impairment can occur in the absence of subjective symptoms, and may not be reliably detected by ordinary clinical exam (i.e., less than formal psychomotor testing). While pharmacodynamic tolerance or adaptation to some adverse depressant effects of flurazepam may develop, patients using flurazepam should be cautioned against driving or engaging in other hazardous activities or activities requiring complete mental alertness.
Additive effects occur with concomitant use of other CNS depressants (e.g., other benzodiazepines, opioids, tricyclic antidepressants, alcohol). Downward dose adjustment of flurazepam and concomitant CNS depressants should be considered. The potential for adverse drug interactions continues for several days following discontinuation of flurazepam, until serum levels of psychoactive metabolites decline.Additive effects occur with concomitant use of other CNS depressants (e.g., other benzodiazepines, opioids, tricyclic antidepressants, alcohol). Downward dose adjustment of flurazepam and concomitant CNS depressants should be considered. The potential for adverse drug interactions continues for several days following discontinuation of flurazepam, until serum levels of psychoactive metabolites decline.
Use of flurazepam with other sedative-hypnotics is not recommended. Alcohol generally should not be used during treatment with flurazepam. The risk of next-day psychomotor impairment is increased if flurazepam is taken with less than a full night of sleep remaining (7 to 8 hours); if higher than the recommended dose is taken; if coadministered with other CNS depressants Use of flurazepam with other sedative-hypnotics is not recommended. Alcohol generally should not be used during treatment with flurazepam. The risk of next-day psychomotor impairment is increased if flurazepam is taken with less than a full night of sleep remaining (7 to 8 hours); if higher than the recommended dose is taken; if coadministered with other CNS depressants [see Dosage and Administration (2)].
Because flurazepam can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
5.3 Benzodiazepine Withdrawal Syndrome
Withdrawal symptoms of the barbiturate type have occurred after the discontinuation of benzodiazepines.
5.4 Need to Evaluate for Co-morbid Disorders
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs.
5.5 Severe Anaphylactic or Anaphylactoid Reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including flurazepam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis.
Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with flurazepam should not be rechallenged with the drug.
5.6 Abnormal Thinking and Behavior Changes
Abnormal thinking and behavior changes have been reported in patients treated with sedative-hypnotics including flurazepam. Some of these changes include decreased inhibition (e.g., aggressiveness and extroversion that seemed out of character), bizarre behavior, and depersonalization. Visual and auditory hallucinations have also been reported. Amnesia, and other neuro-psychiatric symptoms, may occur.
Paradoxical reactions such as stimulation, agitation, increased muscle spasticity, and sleep disturbances may occur unpredictably.
Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake, with amnesia for the event) have been reported with use of sedative-hypnotics. These behaviors can occur with initial treatment or in patients previously tolerant of flurazepam or other sedative-hypnotics. Although these behaviors can occur with use at therapeutic doses, risk is increased by higher doses or concomitant use of alcohol or other CNS depressants. Due to risk to the patient and community, flurazepam should be discontinued if “sleep-driving” occurs.
Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
-
6 ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Risks from Concomitant Use with Opioids [see Warnings and Precautions (5.1)]
- CNS-depressant effects and next-day impairment [see Warnings and Precautions (5.2)]
- Benzodiazepine Withdrawal Syndrome [see Warnings and Precautions (5.3)]
- Severe Anaphylactic and Anaphylactoid Reactions [see Warnings and Precautions (5.4)]
- Abnormal thinking and behavior changes, and complex behaviors [see Warnings and Precautions (5.5)]
- Worsening of depression [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Reported were headache, heartburn, upset stomach, nausea, vomiting, diarrhea, constipation, gastrointestinal pain, nervousness, talkativeness, apprehension, irritability, weakness, palpitations, chest pains, body and joint pains, and genitourinary complaints. There have also been rare occurrences of leukopenia, granulocytopenia, sweating, flushes, difficulty in focusing, blurred vision, burning eyes, faintness, hypotension, shortness of breath, pruritus, skin rash, dry mouth, bitter taste, excessive salivation, anorexia, euphoria, depression, slurred speech, confusion, restlessness, hallucinations and elevated SGOT, SGPT, total and direct bilirubin elevations, and elevated alkaline phosphatase.
-
7 DRUG INTERACTIONS
Benzodiazepines, including flurazepam, produce additive CNS depressant effects when co-administered with ethanol or other CNS depressants (e.g., psychotropic medications, anticonvulsants, antihistamines). Downward dose adjustment of flurazepam and/or concomitant CNS depressants may be necessary because of additive effects.
7.1 Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites, and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists. Limit dosage and duration of concomitant use of benzodiazepines and opioids, and follow patients closely for respiratory depression and sedation.
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Teratogenic Effects. Pregnancy Category C
There are no adequate and well-controlled studies in pregnant women. Available human data on the risk of teratogenicity for benzodiazepines are inconclusive. There is insufficient evidence in humans to assess the effect of benzodiazepine exposure during pregnancy or neurodevelopment. Administration of benzodiazepines immediately prior to or during childbirth can result in a syndrome of hypothermia, hypotonia, respiratory depression, and difficulty feeding. In addition, infants born to mothers who have taken benzodiazepines during the later stages of pregnancy can develop dependence, and subsequently withdrawal, during the postnatal period. Administration of flurazepam to pregnant animals did not indicate a risk for adverse effects on morphological development at clinically relevant doses; however, animal data for other benzodiazepines suggest that possibility of adverse developmental effects (including long-term effects on neurobehavioral and immunological function) following prenatal exposure. Flurazepam should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Pregnancy Registry
To provide information regarding the effects of in utero exposure to flurazepam, physicians are advised to recommend that pregnant patients taking flurazepam enroll in the North American Antiepileptic Drug (NAAED) Pregnancy Registry. This can be done by calling the toll free number 1-888-233-2334, and must be done by patients themselves or their caregiver. Information on the registry can also be found at the website http://www.aedpregnancyregistry.org/.
8.3 Geriatric Use
Flurazepam may cause confusion and over-sedation in the elderly. Elderly patients generally should be started on a low dose of flurazepam and observed closely.
Elderly or debilitated patients may be more sensitive to benzodiazepines, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
9 DRUG ABUSE AND DEPENDENCE
9.1 Controlled Substance
Flurazepam hydrochloride is classified as a Schedule IV controlled substance by federal regulation.
9.2 Abuse and Dependence
Addiction-prone individuals (e.g., history of drug addiction or alcoholism) should be under careful surveillance when receiving flurazepam because of increased risk of abuse and dependence. Benzodiazepine withdrawal symptoms can occur following discontinuation of flurazepam [see Warnings and Precautions (5.2)].
Abuse and addiction are separate and distinct from physical dependence and tolerance. Abuse is characterized by misuse of the drug for non-medical purposes, often in combination with other psychoactive substances. Physical dependence is a state of adaptation that is manifested by a specific withdrawal syndrome that can be produced by abrupt cessation, rapid dose reduction, decreasing blood level of the drug and/or administration of an antagonist. Tolerance is a state of adaptation in which exposure to a drug induces changes that result in a diminution of one or more of the drug’s effects over time. Tolerance may occur to both the desired and undesired effects of drugs and may develop at different rates for different effects.
Addiction is a primary, chronic, neurobiological disease with genetic, psychosocial, and environmental factors influencing its development and manifestations. It is characterized by behaviors that include one or more of the following: impaired control over drug use, compulsive use, continued use despite harm, and craving. Drug addiction is a treatable disease, utilizing a multidisciplinary approach, but relapse is common.
-
10 OVERDOSAGE
Manifestations of flurazepam hydrochloride overdosage include somnolence, confusion and coma. Respiration, pulse and blood pressure should be monitored as in all cases of drug overdosage. General supportive measures should be employed, along with immediate gastric lavage. Intravenous fluids should be administered and an adequate airway maintained. Hypotension and CNS depression may be combated by judicious use of appropriate therapeutic agents. The value of dialysis has not been determined. If excitation occurs in patients following flurazepam hydrochloride overdosage, barbiturates should not be used. As with the management of intentional overdosage with any drug, it should be borne in mind that multiple agents may have been ingested.
Flumazenil, a specific benzodiazepine-receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines and may be useful in situations when an overdose with a benzodiazepine is known or suspected. Prior to the administration of flumazenil, necessary measures should be instituted to secure airway, ventilation, and intravenous access. Flumazenil is intended as an adjunct to, not as a substitute for, proper management of benzodiazepine overdose. Patients treated with flumazenil should be monitored for resedation, respiratory depression, and other residual benzodiazepine effects for an appropriate period after treatment. The prescriber should be aware of a risk of seizure in association with flumazenil treatment, particularly in long-term benzodiazepine users and in cyclic antidepressant overdose. The complete flumazenil package insert including CONTRAINDICATIONS, WARNINGS, and PRECAUTIONS should be consulted prior to use.
-
11 DESCRIPTION
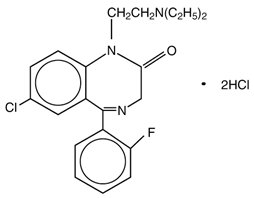
Flurazepam hydrochloride is chemically 7-chloro-1-[2-(diethylamino)ethyl]-5-( o-fluoro-phenyl)-1,3-dihydro-2 H-1,4-benzodiazepin-2-one dihydrochloride. It is a pale yellow, crystalline compound, freely soluble in alcohol and very soluble in water. It has a molecular weight of 460.81 and the following structural formula:
Each capsule for oral administration contains either 15 mg or 30 mg of flurazepam hydrochloride, USP and the following inactive ingredients: colloidal silicon dioxide, FD&C Blue No. 1, FD&C Red No. 3, gelatin, magnesium stearate, microcrystalline cellulose, powdered cellulose, sodium lauryl sulfate and titanium dioxide. The imprinting ink contains black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, propylene glycol and shellac glaze.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Flurazepam, like other central nervous system agents of the 1,4-benzodiazepine class, presumably exerts its effects by binding to stereo-specific receptors at several sites within the central nervous system (CNS). The exact mechanism of action is unknown.
12.3 Pharmacokinetics
Flurazepam hydrochloride is rapidly absorbed from the gastro-intestinal tract. Flurazepam is rapidly metabolized and is excreted primarily in the urine. Following a single oral dose, peak flurazepam plasma concentrations ranging from 0.5 to 4.0 ng/mL occur at 30 to 60 minutes post-dosing. The harmonic mean apparent half-life of flurazepam is 2.3 hours. The blood level profile of flurazepam and its major metabolites was determined in man following the oral administration of 30 mg daily for 2 weeks. The N1-hydroxyethyl-flurazepam was measurable only during the early hours after a 30 mg dose and was not detectable after 24 hours. The major metabolite in blood was N1-desalkyl-flurazepam, which reached steady-state (plateau) levels after 7 to 10 days of dosing, at levels approximately 5- to 6-fold greater than the 24-hour levels observed on Day 1. The half-life of elimination of N1-desalkyl-flurazepam ranged from 47 to 100 hours. The major urinary metabolite is conjugated N1-hydroxyethyl-flurazepam which accounts for 22% to 55% of the dose. Less than 1% of the dose is excreted in the urine as N1-desalkyl-flurazepam.
This pharmacokinetic profile may be responsible for the clinical observation that flurazepam is increasingly effective on the second or third night of consecutive use and that for 1 or 2 nights after the drug is discontinued both sleep latency and total wake time may still be decreased.
The single dose pharmacokinetics of flurazepam were studied in 12 healthy geriatric subjects (aged 61 to 85 years). The mean elimination half-life of desalkyl-flurazepam was longer in elderly male subjects (160 hours) compared with younger male subjects (74 hours), while mean elimination half-life was similar in geriatric female subjects (120 hours) and younger female subjects (90 hours). After multiple dosing, mean steady-state plasma levels of desalkyl-flurazepam were higher in elderly male subjects (81 ng/mL) compared with younger male subjects (53 ng/mL), while values were similar between elderly female subjects (85 ng/mL) and younger female subjects (86 ng/mL). The mean washout half-life of desalkyl-flurazepam was longer in elderly male and female subjects (126 and 158 hours, respectively) compared with younger male and female subjects (111 and 113 hours, respectively). 1
1 Greenblatt DJ, Divoll M, Hammatz JS, MacLauglin DS, Shader RI: Kinetics and clinical effects of flurazepam in young and elderly noninsomniacs. Clin Pharmacol Ther 30:475–486, 1981.
- 13 NONCLINICAL TOXICOLOGY
- 14 CLINICAL STUDIES
-
16 HOW SUPPLIED/STORAGE AND HANDLING
Flurazepam Hydrochloride Capsules, USP are available containing either 15 mg or 30 mg of flurazepam hydrochloride, USP.
The 30 mg capsule is a hard-shell gelatin capsule with a powder blue opaque cap and a powder blue opaque body filled with off-white to yellow powder. The capsule is axially printed with MYLAN over 4430 in black ink on both the cap and body. They are available as follows:
NDC: 55289-038-30
bottles of 30 capsulesStore at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from light.
Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
PHARMACIST: Dispense a Medication Guide with each prescription.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling ( Medication Guide).
Inform patients about the benefits and risks of flurazepam, stressing the importance of use as directed. Assist patients in understanding the Medication Guide and instruct them to read it with each prescription refill.
Inform patients and caregivers that potentially fatal additive effects may occur if flurazepam is used with opioids and not to use such drugs concomitantly unless supervised by a health care provider [see Warnings and Precautions (5.1), Drug Interactions (7.1)] .
CNS Depressant Effects and Next-Day Impairment: Tell patients that flurazepam can cause next-day impairment, even in the absence of symptoms. Caution patients against driving or engaging in other hazardous activities or activities requiring complete mental alertness when using flurazepam. Tell patients that daytime impairment may persist for several days following discontinuation of flurazepam. Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients.
Withdrawal: Instruct patients to contact you before stopping or decreasing the dose of flurazepam, because withdrawal symptoms can occur.
Abnormal Thinking and Behavior Change: Instruct patients that sedative hypnotics can cause abnormal thinking and behavior change, including “sleepdriving” and other complex behaviors while not being fully awake (preparing and eating food, making phone calls, or having sex). Tell patients to call you immediately if they develop any of these symptoms.
Severe Allergic Reactions: Inform patients that severe allergic reactions can occur from flurazepam. Describe the signs/symptoms of these reactions and advise patients to seek medical attention immediately if these occur.
Suicide: Tell patients that flurazepam can worsen depression, and to immediately report any suicidal thoughts.
Alcohol and Other Drugs: Ask patients about alcohol consumption, medicines they are taking now, and drugs they may be taking without a prescription. Advise patients that alcohol generally should not be used during treatment with flurazepam.
Pregnancy: Instruct patients to inform you if they are nursing or pregnant, or may become pregnant while taking flurazepam. If a woman becomes pregnant while taking flurazepam, she should discontinue use immediately.
Tolerance, Abuse, and Dependence: Tell patients not to increase the dose of flurazepam on their own, and to inform you if they believe the drug “does not work”.
-
Medication Guide
Flurazepam Hydrochloride Capsules, USP
(flur az' e pam hye" droe klor' ide)
What is the most important information I should know about flurazepam hydrochloride capsules?
- Flurazepam hydrochloride capsules are a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death.
- Flurazepam hydrochloride capsules may cause serious side effects that you may not know are happening to you. These side effects include:
- sleepiness during the day
- not thinking clearly
- act strangely, confused, or upset
- “sleep-walking” or doing other activities when you are asleep like:
- eating
- talking
- having sex
- driving a car
Call your healthcare provider right away if you find out that you have done any of the above activities after taking flurazepam hydrochloride capsules.
- Do not take flurazepam hydrochloride capsules unless you are able to stay in bed a full night (7 to 8 hours) before you must be active again.
- Do not take more flurazepam hydrochloride capsules than prescribed.
What are flurazepam hydrochloride capsules?
- Flurazepam hydrochloride capsules are a prescription medicine used to treat certain types of insomnia including difficulty falling asleep, waking up often during the night, or waking up early in the morning.
- Flurazepam hydrochloride is a federal controlled substance (C-IV) because it can be abused or lead to dependence. Keep in a safe place to prevent misuse and abuse. Selling or giving away flurazepam hydrochloride capsules may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
- It is not known if flurazepam hydrochloride capsules are safe and effective in children.
Do not take flurazepam hydrochloride capsules if you:
- are allergic to flurazepam hydrochloride, other benzodiazepines, or any of the ingredients in flurazepam hydrochloride capsules. See the end of this Medication Guide for a complete list of ingredients in flurazepam hydrochloride capsules. Symptoms of a serious allergic reaction can include:
- swelling of your face, lips, and throat that may cause difficulty breathing or swallowing
- nausea and vomiting
Before you take flurazepam hydrochloride capsules, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of depression, mental illness or, suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- are pregnant or planning to become pregnant. Flurazepam hydrochloride may harm your unborn baby. You and your healthcare provider should decide if you should take flurazepam hydrochloride capsules while you are pregnant.
- are breastfeeding, or plan to breastfeed. Flurazepam hydrochloride may pass through your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take flurazepam hydrochloride capsules.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Taking flurazepam hydrochloride capsules with certain other medicines can cause side effects or affect how well flurazepam hydrochloride capsules or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider.
Do not take flurazepam hydrochloride capsules with other medicines that can make you sleepy unless your healthcare provider tells you to.
How should I take flurazepam hydrochloride capsules?
- See “What is the most important information I should know about flurazepam hydrochloride capsules?”
- Take flurazepam hydrochloride capsules exactly as your healthcare provider tells you to take it.
- Take flurazepam hydrochloride capsules right before you get into bed.
- Do not take flurazepam hydrochloride capsules unless you are able to get a full night’s sleep before you must be active again.
- If you take too many flurazepam hydrochloride capsules, get emergency treatment right away.
What should I avoid while taking flurazepam hydrochloride capsules?
- You may still feel drowsy the next day after taking flurazepam hydrochloride capsules. Do not drive, operate machinery, do other dangerous activities or do anything that needs you to be alert until you know how flurazepam hydrochloride capsules affect you.
- You should not drink alcohol while you are taking flurazepam hydrochloride capsules.
What are the possible side effects of flurazepam hydrochloride capsules?
Flurazepam hydrochloride capsules may cause serious side effects, including:
- See “What is the most important information I should know about flurazepam hydrochloride capsules?”
- Withdrawal symptoms. You may have withdrawal symptoms if you stop taking flurazepam hydrochloride capsules suddenly. Withdrawal symptoms can be serious and include seizures. Mild withdrawal symptoms include a depressed mood and trouble sleeping. Talk to your healthcare provider about slowly stopping flurazepam hydrochloride capsules to avoid withdrawal symptoms.
- Other conditions. Call your healthcare provider if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- Severe allergic reactions. Symptoms include swelling of the tongue or throat, and trouble breathing. Other symptoms may include nausea and vomiting. Get emergency medical help right away if you have these symptoms after taking flurazepam hydrochloride capsules.
- Abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, and suicidal thoughts.
- Depression. Pre-existing depression may emerge or worsen during use of benzodiazepines including flurazepam hydrochloride capsules.
- Abuse and dependence. Taking flurazepam hydrochloride capsules can cause physical and psychological dependence. Physical and psychological dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical and psychological dependence and drug addiction.
The most common side effects of flurazepam hydrochloride capsules include:
- dizziness
- light-headedness
- loss of coordination
- drowsiness
- staggering
- falling
These are not all the possible side effects of flurazepam hydrochloride capsules. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store flurazepam hydrochloride capsules?
- Store at 20° to 25°C (68° to 77°F).
- Keep flurazepam hydrochloride capsules in a tightly closed container and out of the light.
Keep flurazepam hydrochloride capsules and all medicines out of the reach of children.
General information about the safe and effective use of flurazepam hydrochloride capsules.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use flurazepam hydrochloride capsules for a condition for which it was not prescribed. Do not give flurazepam hydrochloride capsules to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about flurazepam hydrochloride capsules that is written for healthcare professionals.
What are the ingredients in flurazepam hydrochloride capsules?
Active Ingredient: flurazepam hydrochloride
Inactive Ingredients: colloidal silicon dioxide, FD&C Blue No. 1, FD&C Red No. 3, gelatin, magnesium stearate, microcrystalline cellulose, powdered cellulose, sodium lauryl sulfate and titanium dioxide. The imprinting ink contains black iron oxide, D&C Yellow No. 10 Aluminum Lake, FD&C Blue No. 1 Aluminum Lake, FD&C Blue No. 2 Aluminum Lake, FD&C Red No. 40 Aluminum Lake, propylene glycol and shellac glaze.
Manufactured by: Mylan Pharmaceuticals Inc., Morgantown, WV 26505 U.S.A.
If you would like more information, call Mylan at 1-877-446-3679 (1-877-4-INFO-RX).
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.Revised: 12/2018
FLZ:R21m/MG:FLZ:R6m - PRINCIPAL DISPLAY PANEL – 30 mg
-
INGREDIENTS AND APPEARANCE
FLURAZEPAM HYDROCHLORIDE
flurazepam hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 55289-038(NDC:0378-4430) Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURAZEPAM HYDROCHLORIDE (UNII: 756RDM536M) (FLURAZEPAM - UNII:IHP475989U) FLURAZEPAM HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE 102 (UNII: PNR0YF693Y) POWDERED CELLULOSE (UNII: SMD1X3XO9M) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FERROSOFERRIC OXIDE (UNII: XM0M87F357) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) Product Characteristics Color blue (powder blue opaque) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code MYLAN;4430 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55289-038-30 30 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 05/06/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA070345 11/27/1985 Labeler - PD-Rx Pharmaceuticals, Inc. (156893695) Registrant - PD-Rx Pharmaceuticals, Inc. (156893695) Establishment Name Address ID/FEI Business Operations PD-Rx Pharmaceuticals, Inc. 156893695 repack(55289-038)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.