BRONCOMAR- acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl liquid
Broncomar by
Drug Labeling and Warnings
Broncomar by is a Otc medication manufactured, distributed, or labeled by Gadal Laboratories Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
ACTIVE INGREDIENT
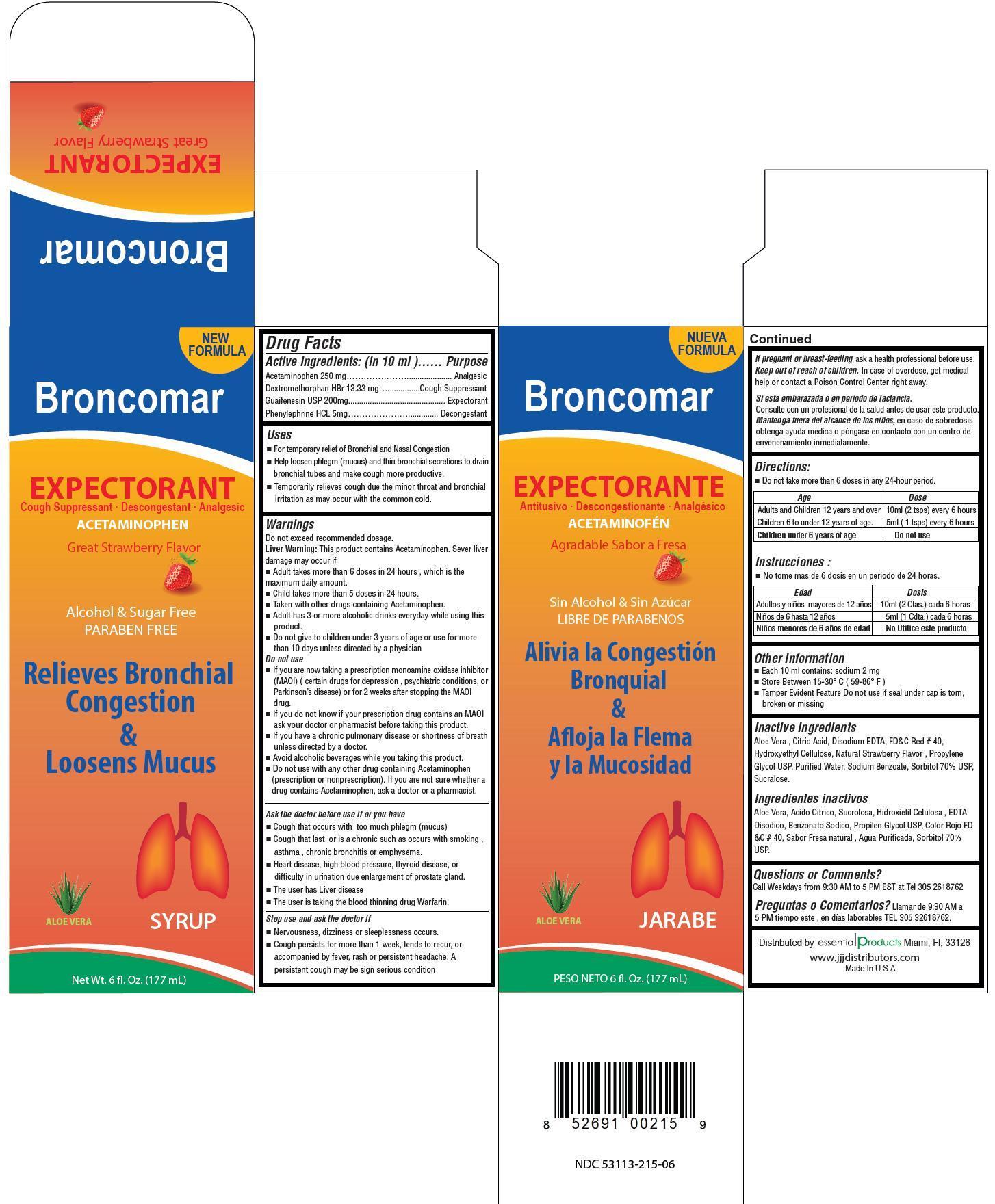
Acvtive ingredients: (in 10 ml) Purpose
Acetaminophen 200 mg ............................. Analgesic
Dextromethorphan HBr 13.33 mg ................... Cough Suppressant
Guaifenesin 200 mg ................................... Expectorant
Phenylephrine HCl 5 mg ............................. Decongestant
- PURPOSE
- INDICATIONS & USAGE
-
WARNINGS
Warnings
Do not exceed recommended dosage
Liver warning: This product contains Acetaminophen. Sever liver damage may occur if
- Adult takes more than 6 doses in 24 hours, which is the maximum daily amount
- Child takes more than 5 doses in 24 hours
- Taken with other drugs containing Acetaminophen
- Adult has 3 or more alcoholic drinks everyday while using this product
- Do not give to children under 3 years of age or use for more than 10 days unless directed by a physician
-
DO NOT USE
Do not use
- If you are now taking a prescription monoamine oxidase inhibitor (MAOI)( certain drugs for depression , psychiatric conditions, or Parkinson’s disease) or for 2 weeks after stopping the MAOI drug.
- if you do not know if your prescription drug contains on MAOI ask your doctor or pharmacist before taking this product
- if you have a chronic pulmonary disease or shortness of breath unless directed by a doctor
- avoid alcoholic beverages while you are taking this product
- do not use with any other drug containing Acetaminophen (prescirption or nonprescription). If you are not sure whether a drug contains Acetaminophen, ask a doctor or a pharmacist.
Ask the doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
- heart disease, high blood pressure, thyroid disease, or difficulty in urination due to the enlargement of prostate gland.
- The user has liver disease
- The user is taking the blood thinning drug Warfarin.
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BRONCOMAR
acetaminophen, dextromethorphan hbr, guaifenesin, phenylephrine hcl liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53113-215 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 250 mg in 10 mL DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 13.33 mg in 10 mL GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 5 mg in 10 mL Inactive Ingredients Ingredient Name Strength ALOE VERA WHOLE (UNII: KIZ4X2EHYX) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYMETHYL CELLULOSE (UNII: 273FM27VK1) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53113-215-06 1 in 1 CARTON 1 177 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 06/01/2014 Labeler - Gadal Laboratories Inc (841305639)
Trademark Results [Broncomar]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BRONCOMAR 86169790 4543652 Live/Registered |
J.J.J DISTRIBUTORS 2014-01-20 |
 BRONCOMAR 73043470 1026059 Dead/Expired |
MARLOP PHARMACEUTICALS, INC. 1975-02-05 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.