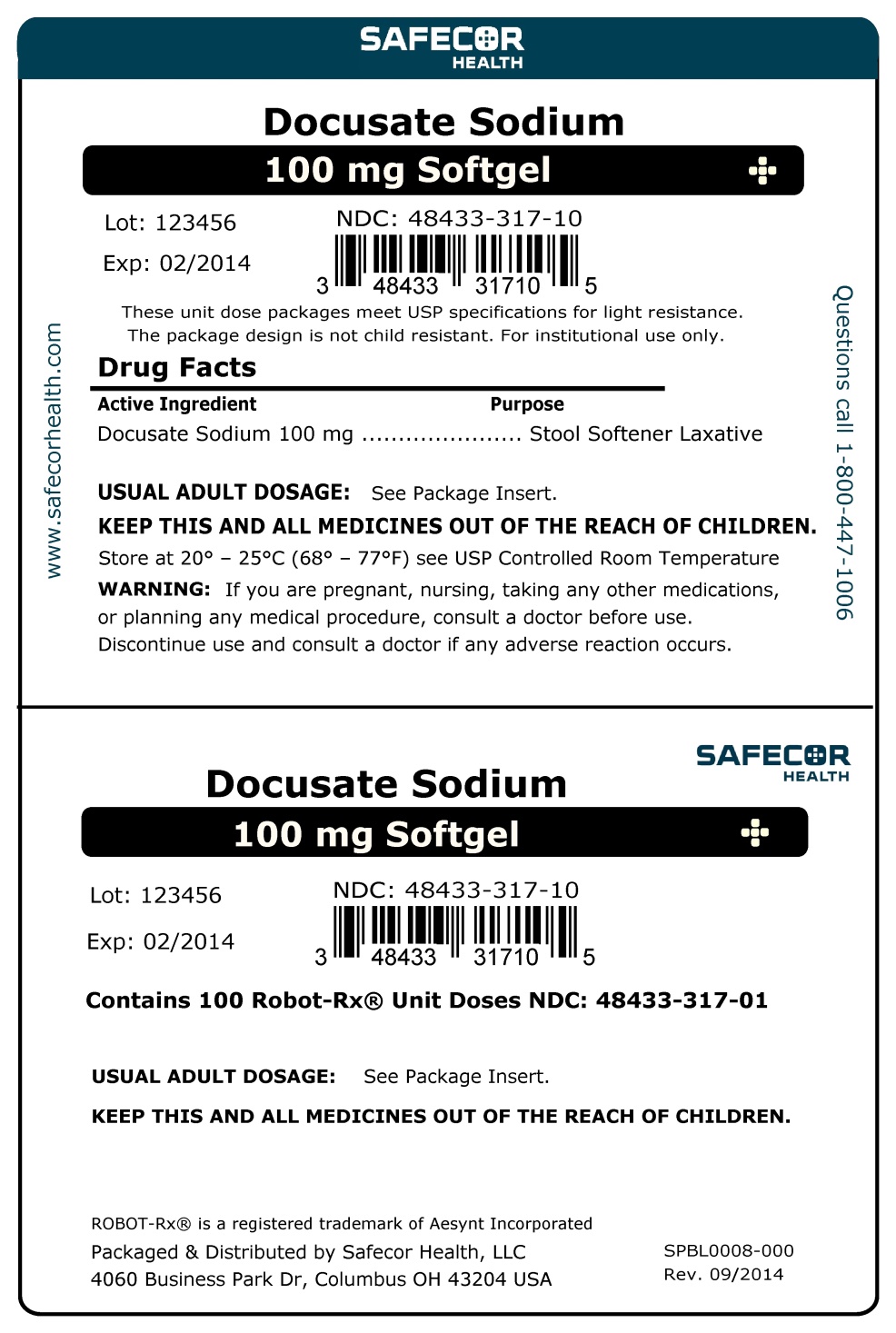
Docusate Sodium, USP Stool Softener
Docusate Sodium by
Drug Labeling and Warnings
Docusate Sodium by is a Otc medication manufactured, distributed, or labeled by Safecor Health, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
DOCUSATE SODIUM- docusate sodium capsule, liquid filled
Safecor Health, LLC
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
Docusate Sodium, USP
Stool Softener
Keep Out of Reach of Children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Uses
- For the relief of occasional constipation.
- Helps to prevent dry, hard stools.
- This product generally produces a bowel movement within 12 to 72 hours.
Ask a doctor before use
if you notice a sudden change in bowel habits that persists over a period of two weeks.
Stop use and ask a doctor
if you have rectal bleeding or you fail to have a bowel movement after use.
Directions
|
Adults and Children over 12 years of age |
Take orally 1 to 2 softgels preferably at bedtime for |
|
Children 6 to 12 years of age |
Take orally 1 softgel preferably at bedtime for |
|
Children under 6 years of age |
Do not use this product for children under 6 years of age, unless directed by a doctor. |
Other Information
- Each softgel contains 5 mg of Sodium.
- Store at room temperature between 15°C to 30°C (59°F to 86°F).
- Do not use if seal on packaging is open or missing.
Rev: 317-00 08/2014
| DOCUSATE SODIUM
docusate sodium capsule, liquid filled |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Safecor Health, LLC (828269675) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Safecor Health, LLC | 828269675 | REPACK(48433-317) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Safecor Health, LLC | 078805287 | REPACK(48433-317) | |