ENTYCE- capromorelin tartrate solution
Entyce by
Drug Labeling and Warnings
Entyce by is a Animal medication manufactured, distributed, or labeled by Elanco US Inc., Halo Pharmaceutical Canada, Inc, Cambrex Charles City, Sterling Wisconsin, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
Description:
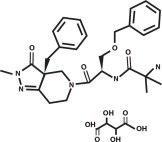
ENTYCE (capromorelin oral solution) is a selective ghrelin receptor agonist that binds to receptors and affects signaling in the hypothalamus to cause appetite stimulation and binds to the growth hormone secretagogue receptor in the pituitary gland to increase growth hormone secretion. The empirical formula is C28H35N5O4·C4H6O6 and the molecular weight 655.70. The chemical name is 2-amino-N-[2-(3aR-benzyl-2-methyl-3-oxo-2,3,3a,4,6,7-hexahydro-pyrazolo[4,3-c]pyridin-5-yl)1R-benzyloxymethyl-2-oxo-ethyl]-isobutyramide L-tartrate.
The chemical structure of capromorelin tartrate is:
- Indication:
-
Dosage and Administration:
Administer ENTYCE orally at a dose of 3 mg/kg (1.4 mg/lb) body weight once daily.
To administer ENTYCE follow the written instructions below and see illustrations 1 through 4 for administration steps.
- 1. Gently shake the bottle.
- 2. Remove the bottle cap and insert the provided dosing syringe firmly into the opening of the bottle.
- 3. Turn the bottle upside down and withdraw the appropriate volume of solution. Return the bottle to the upright position before removing the syringe, and replace the cap.
- 4. Administer the solution with the syringe into the dog's mouth.
Rinse the syringe and plunger with water between treatment doses. Leave the syringe and plunger apart to dry. Wash hands immediately after use.
The effectiveness of ENTYCE has not been evaluated beyond 4 days of treatment in the clinical field study (See Effectiveness).
- Contraindications:
-
Warnings:
User Safety Warnings:
Not for use in humans. Keep this drug, including used syringes, out of reach of children. Wash hands immediately after use as this product may be dermally absorbed. Consult a physician in case of accidental ingestion by humans. To obtain a Safety Data Sheet(s), contact Elanco US Inc. at 1-888-545-5973.
For use in dogs only.
Animal Safety Warnings:
Keep ENTYCE in a secure location out of reach of dogs, cats, and other animals to prevent accidental ingestion or overdose.
-
Precautions:
Use with caution in dogs with hepatic dysfunction. ENTYCE is metabolized by CYP3A4 and CYP3A5 enzymes (See Clinical Pharmacology).
Use with caution in dogs with renal insufficiency. ENTYCE is excreted approximately 37% in urine and 62% in feces (See Adverse Reactions and Clinical Pharmacology).
Use with caution in dogs that may have cardiac disease or severe dehydration. ENTYCE can cause transient decreases in heart rate and blood pressure following dose administration. Some dogs may exhibit clinical signs of bradycardia or hypotension following administration of ENTYCE (See Post-Approval Experience).
Use with caution in dogs with diabetes mellitus as hyperglycemia has been reported following administration of ENTYCE (See Post-Approval Experience).
The safe use of ENTYCE has not been evaluated in dogs used for breeding or pregnant or lactating bitches.
-
Adverse Reactions:
In a controlled field study, 244 dogs were evaluated for safety when administered either ENTYCE or a vehicle control (solution minus capromorelin) at a dose of 3 mg/kg once daily for 4 days. Enrolled dogs had a reduced or absent appetite for a minimum of 2 days prior to day 0 and had various medical conditions: arthritis (40); gastrointestinal disease (24); allergy (22); dental disease (22); cardiovascular disease (16); renal disease (13); and others. Some dogs may have experienced more than one of the adverse reactions during the study.
The following adverse reactions were observed:
Table 1: Adverse Reactions reported in dogs administered ENTYCE oral solution compared to vehicle control Adverse Reactions
ENTYCE (n = 171)
n (%)Vehicle Control (n = 73)
n (%)GASTROINTESTINAL
Diarrhea
12 (7.0 %)
5 (6.8 %)
Vomiting
11 (6.4 %)
4 (5.5 %)
Hypersalivation
4 (2.3 %)
0 (0.0 %)
Abdominal discomfort
2 (1.2 %)
0 (0.0 %)
Flatulence
2 (1.2 %)
0 (0.0 %)
Nausea
2 (1.2 %)
0 (0.0 %)
CLINICAL PATHOLOGY
Elevated blood urea nitrogen
7 (4.1 %)
2 (2.7 %)
Elevated phosphorus
4 (2.3 %)
1 (1.4 %)
Elevated creatinine
1 (0.6 %)
1 (1.4 %)
OTHER
Polydipsia
7 (4.1 %)
1 (1.4 %)
Lethargy/depression
2 (1.2 %)
0 (0.0 %)
The following adverse reactions were reported in < 1% of dogs administered ENTYCE: hyperactivity, increase fecal volume, increase gut sounds, and polyuria.
Post-Approval Experience (2025):
The following adverse events are based on post-approval adverse drug experience reporting for ENTYCE. Not all adverse events are reported to FDA/CVM. It is not always possible to reliably estimate the adverse event frequency or establish a causal relationship to product exposure using these data.
The following adverse events in dogs are categorized in order of decreasing reporting frequency by body system and in decreasing order of reporting frequency within each body system:
Gastrointestinal: vomiting, hypersalivation, diarrhea
General: lethargy, polydipsia, weakness, hyperglycemia, recumbency
Behavioral: Unusual behaviors (some of these behaviors were related to avoidance of medication), vocalization, hyperactivity
Neurological: ataxia, loss of consciousness, disorientation, sedation
Respiratory: panting, dyspnea
Cardiovascular: bradycardia, hypotension
-
Contact Information:
To report suspected adverse drug experiences, contact Elanco US Inc. at 1-888-545-5973.
For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or http://www.fda.gov/reportanimalae
-
Clinical Pharmacology:
Following oral administration of ENTYCE at a dose of 3 mg/kg to 12 Beagle dogs, absorption of capromorelin was rapid with the maximum concentration (Cmax) reached within 0.83 hr (Tmax). After Cmax, the plasma concentrations declined mono-exponentially with a short terminal half-life (T½) of approximately 1.19 hrs. There were no gender differences in capromorelin pharmacokinetics. The exposure (Cmax and AUC) of capromorelin increased with dose, but the increases were not dose proportional following single and repeat once daily administrations of capromorelin. There was no drug accumulation following repeat oral administration.
Table 2. Plasma PK parameters following oral administration of 3 mg/kg of ENTYCE Parameter
Mean
SD
Tmax (hr)
0.83
0.58
Cmax (ng/mL)
330
143
AUCt (ng*hr/mL)
655
276
AUCinf (ng*hr/mL)
695
262
T½ (hr)
1.19
0.17
The mean absolute oral bioavailability of capromorelin was 44%. The mean total plasma clearance and volume of distribution was 18.9 mL/min/kg and 2.0 L/kg, respectively. Capromorelin was not highly bound (unbound fraction 51%) to plasma protein. The protein binding was concentration-independent over the range of 10 to 1000 ng/mL. In vitro (human liver microsomes) and in vivo (rats) metabolism studies suggest that capromorelin is metabolized by hepatic enzymes, mainly CYP3A4 and CYP3A5. Therefore, drugs that inhibit CYP3A4 and CYP3A5 activity may affect capromorelin metabolism. Following oral administration of radio-labelled capromorelin to dogs, capromorelin was excreted in urine (37%) and in feces (62%) within 72 hours.
-
Effectiveness:
Laboratory Effectiveness Study: Twenty four healthy Beagle dogs (6 dogs per sex in each group) with normal appetite were randomized into two groups and dosed daily with ENTYCE (capromorelin oral solution) at 3 mg/kg/day or vehicle control (solution minus capromorelin) to compare food intake over a 4-day period. The dogs were 13 months of age and weighed between 6.5 and 12.5 kg at the time of randomization. Six dogs administered ENTYCE repeatedly exhibited salivation post dosing and two dogs administered vehicle control exhibited salivation only one time on study day 0. Emesis was observed in one dog administered ENTYCE on study day 1. Dogs administered ENTYCE at a dose of 3 mg/kg/day for 4 consecutive days had statistically significantly increased food consumption compared to the vehicle control group (p < 0.001).
Clinical Field Study: Effectiveness was evaluated in 177 dogs (121 dogs in the ENTYCE group and 56 dogs in the vehicle control group) in a double-masked, vehicle controlled field study. Dogs with a reduced appetite or no appetite, with various medical conditions, for a minimum of 2 days prior to day 0 were enrolled in the study. The dogs ranged in age from 4 months to 18 years. Dogs were randomized to treatment group and dosed once daily for 4 days with ENTYCE at 3 mg/kg or vehicle control. Dogs were assessed for appetite by owners on day 0 and day 3 ± 1 using an “increased”, “no change” or “decreased” scoring system. Dogs were classified as a treatment success if the owner scored their dog's appetite as “increased” on day 3 ± 1. The success rates of the two groups were significantly different (p = 0.0078); 68.6% (n = 83) of dogs administered ENTYCE were successes, compared to 44.6% (n = 25) of the dogs in the vehicle control group.
-
Target Animal Safety:
In a 12-month laboratory safety study, 32 healthy Beagle dogs (4 dogs per sex per group) approximately 11-12 months of age and weighing 9-13.6 kg were dosed orally with capromorelin in deionized water daily at 0X (placebo), 0.3 (0.13X), 7 (3.07X), and 40 (17.5X) mg/kg/day. Administration of capromorelin was associated with increased salivation and reddening/swollen paws, increased liver weights and hepatocellular cytoplasmic vacuolation. Treatment related decreases were seen in red blood cell count, hemoglobin and hematocrit in the 40 mg/kg group. Pale skin, pale gums, and decreased red blood cell count, hemoglobin and hematocrit were observed in one dog administered 40 mg/kg/day. Increases were seen in cholesterol, high density lipoproteins, and the liver specific isozyme of serum alkaline phosphatase in the 40 mg/kg group. Growth hormone and insulin-like growth factor 1 plasma levels were increased in all groups administered capromorelin. There were no effects noted on gross necropsy. Capromorelin levels were similar in plasma collected on days 90, 181, and 349 indicating no accumulation of drug.
- Storage Conditions:
-
How Supplied:
30 mg/mL flavored solution in 10 mL, 15 mL and 30 mL bottles with measuring syringe
Approved by FDA under NADA # 141-457
Manufactured for:
Elanco US Inc.
Indianapolis, IN 46221 USARevised: August 2025
ENTYCE, Elanco and the diagonal bar logo are trademarks of Elanco or its affiliates.
© 2025 Elanco or its affiliates
ElancoTM
PA104292X - Principal Display Panel - 10 mL Carton Label
- Principal Display Panel - 10 mL Bottle Label
- Principal Display Panel - 15 mL Carton Label
- Principal Display Panel - 15 mL Bottle Label
- Principal Display Panel - 30 mL Carton Label
- Principal Display Panel - 30 mL Bottle Label
-
INGREDIENTS AND APPEARANCE
ENTYCE
capromorelin tartrate solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 58198-5535 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Capromorelin tartrate (UNII: 4150VMF5EP) (Capromorelin - UNII:0MQ44VUN84) Capromorelin tartrate 30 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 58198-5535-1 1 in 1 CARTON 1 10 mL in 1 BOTTLE, PLASTIC 2 NDC: 58198-5535-2 1 in 1 CARTON 2 15 mL in 1 BOTTLE, PLASTIC 3 NDC: 58198-5535-3 1 in 1 CARTON 3 30 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NADA NADA141457 02/08/2017 Labeler - Elanco US Inc. (966985624)
Trademark Results [Entyce]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ENTYCE 86976155 4757702 Live/Registered |
Aratana Therapeutics, Inc. 2014-04-29 |
 ENTYCE 86976147 4745269 Live/Registered |
ARATANA THERAPEUTICS, INC. 2013-09-30 |
 ENTYCE 86265120 not registered Dead/Abandoned |
Aratana Therapeutics, Inc. 2014-04-29 |
 ENTYCE 86078877 not registered Dead/Abandoned |
ARATANA THERAPEUTICS, INC. 2013-09-30 |
 ENTYCE 85908883 not registered Dead/Abandoned |
Red Chillies Inc. 2013-04-19 |
 ENTYCE 77101705 not registered Dead/Abandoned |
Jones, Mary H. 2007-02-07 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.