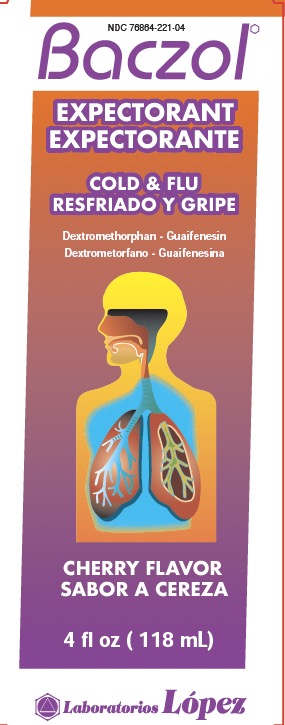
BACZOL EXPECTORANT (G) (DA)
Baczol by
Drug Labeling and Warnings
Baczol by is a Otc medication manufactured, distributed, or labeled by Procaps S.A. de C.V.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
BACZOL EXPECTORANT- dextromethorphan, guaifenesin liquid
Procaps S.A. de C.V.
----------
BACZOL EXPECTORANT (G) (DA)
Active Ingredients & Purposes
Active ingredients (in each 5 mL tsp.)
Guaifenesin 200 mg
Phenylephrine HCl 5 mg
Uses
Temporarily relieves common cold symptoms:
- cough due to minor throat & bronchial irritation
- helps loosen phlegm (mucus) and thin bronchial secretions to make coughs more productive
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if the user has a
- cough that is accompanied by excessive phlegm (mucus)
- persistent or chronic cough such as occurs with smoking, asthma or emphysema
Directions
- only use the dosing cup provided
- do not exceed 6 doses in a 24-hour period
| Age | Dose |
| adults and children 12 years and older | 20mL every 4 hours |
| children 6 to 12 years | 10mL every 4 hours |
| children under 6 years | do not use |
Other information
- each 10 mL contains: potassium 10mg, sodium 10mg
- store between 68-77°F (20-25°C)
- do not refrigerate
Inactive ingredients
citric acid anhydrous, D&C red #33, dextrose, FD&C red #40, flavor, glycerin, methylparaben, potassium sorbate, propylene glycol, propylparaben, purified water, saccharin sodium, sodium hydroxide, sorbitol, sucralose, xanthan gum.
| BACZOL
EXPECTORANT
dextromethorphan, guaifenesin liquid |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Labeler - Procaps S.A. de C.V. (851259341) |
Revised: 12/2024
Document Id: 2a878c11-91ce-87aa-e063-6294a90a5c4b
Set id: 0b66a67f-3b54-e7ed-e063-6294a90a2f32
Version: 3
Effective Time: 20241230
Trademark Results [Baczol]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 BACZOL 87236823 5344510 Live/Registered |
LABORATORIOS LOPEZ, S.A. DE C.V. 2016-11-15 |
 BACZOL 77930773 3872527 Dead/Cancelled |
LABORATORIOS LOPEZ, S.A. DE C.V. 2010-02-08 |
 BACZOL 77894598 not registered Dead/Abandoned |
HEIDBRINK GARED 2009-12-16 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.