FIRSTCARE DEXTROMETHORPHAN HYDROBROMIDE, DOXYLAMINE SUCCINATE by USpharma Ltd Drug Facts
FIRSTCARE DEXTROMETHORPHAN HYDROBROMIDE, DOXYLAMINE SUCCINATE by
Drug Labeling and Warnings
FIRSTCARE DEXTROMETHORPHAN HYDROBROMIDE, DOXYLAMINE SUCCINATE by is a Otc medication manufactured, distributed, or labeled by USpharma Ltd. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FIRSTCARE DEXTROMETHORPHAN HYDROBROMIDE, DOXYLAMINE SUCCINATE- dextromethorphan hydrobromide, doxylamine succinate tablet, chewable
USpharma Ltd
----------
Drug Facts
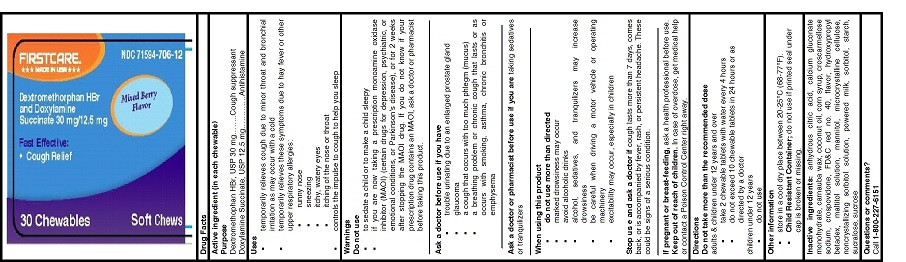
Active ingredients (in each chewable tablet)
Dextromethorphan HBr, USP 30 mg
Doxylamine Succinate, USP 12.5 mg
Uses
- temporarily relieves cough due to minor throat and bronchial irritation as may occur with a cold
- temporarily relieves these symptoms due to hay fever or other upper respiratory allergies:
- runny nose
- sneezing
- itchy, watery eyes
- itching of the nose or throat
- controls the impulse to cough to help you sleep
Warnings
Do not use
- to sedate a child or to make a child sleepy
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- trouble urinating due to an enlarged prostate gland
- glaucoma
- a cough that occurs with too much phlegm (mucus)
- a breathing problem or chronic cough that lasts or as occurs with smoking, asthma, chronic bronchitis or emphysema
When using this product
- do not use more than directed
- marked drowsiness may occur
- avoid alcoholic drinks
- alcohol, sedatives, and tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
Directions
Do not take more than the recommended dose
adults & children under 12 years and over
- take 2 chewable tablets with water every 4 hours
- do not exceed 10 chewable tablets in 24 hours or as directed by a doctor
children under 12 years
- do not use
Other information
- store in a cool dry place between 20-25°C (68-77°F).
- Child Resistant Container;do not use if printed seal under cap is broken or missing.
Inactive ingredients
anhydrous citric acid, calcium gluconate monohydrate, carnauba wax, coconut oil, corn syrup, croscarmellose sodium, crospovidone, FD&C red no. 40, flavor, hydroxypropyl betadex, maltitol solution, mannitol, microcrystalline cellulose, noncrystallizing sorbitol solution, powered milk, sorbitol, starch, sucralose, sucrose
PRINCIPAL DISPLAY PANEL
FIRSTCARETM
***MADE IN USA***
NDC: 71594-706-12
Mixed Berry Flavor
Dextromethorphan HBr and Doxylamine Succinate 30mg/12.5 mg
Fast Effective:Cough Relief
30 Chewables
SOFT CHEWS
| FIRSTCARE DEXTROMETHORPHAN HYDROBROMIDE, DOXYLAMINE SUCCINATE
dextromethorphan hydrobromide, doxylamine succinate tablet, chewable |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - USpharma Ltd (080664601) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| USpharma Ltd | 080664601 | manufacture(71594-706) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.