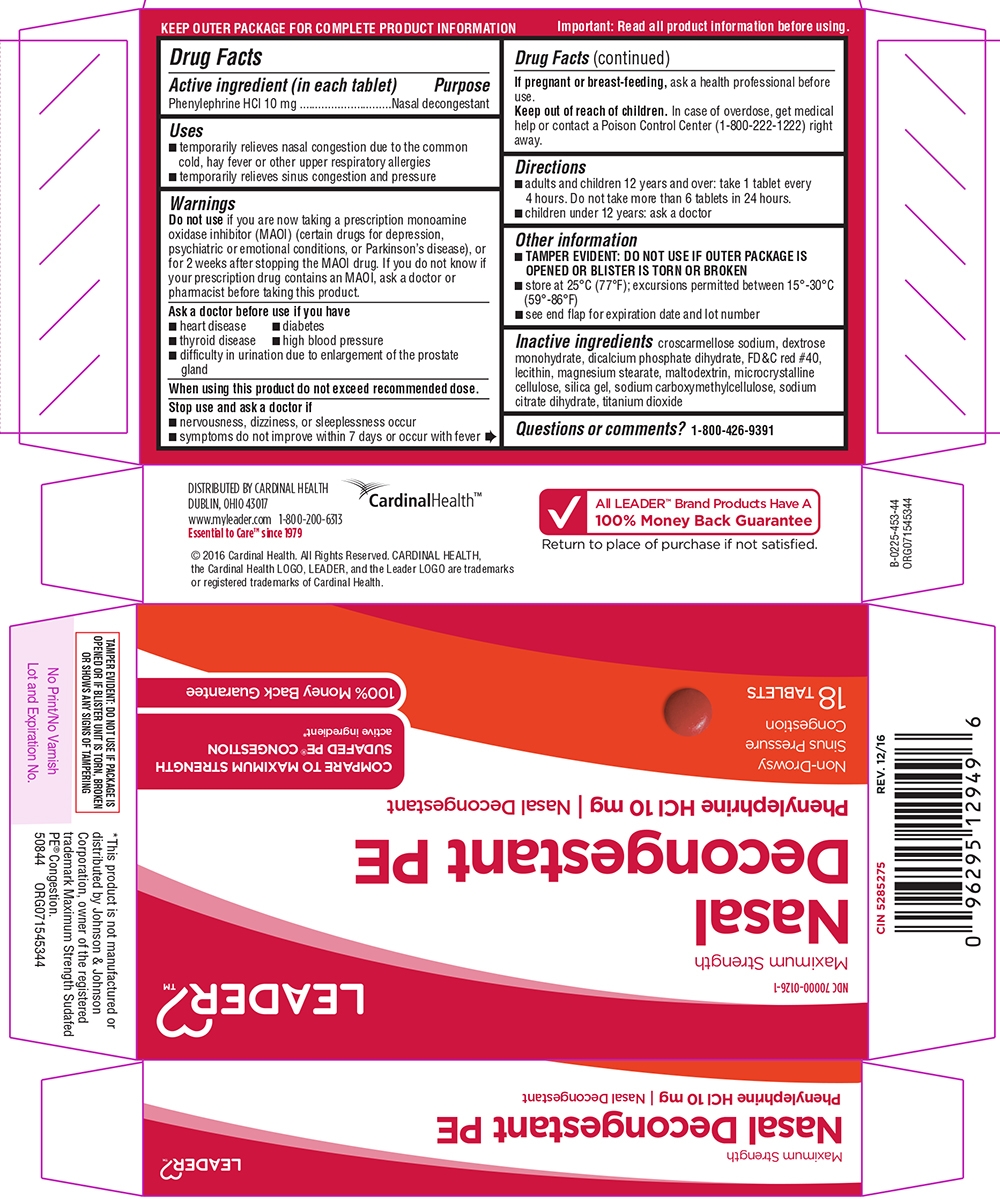
NASAL DECONGESTANT PE MAXIMUM STRENGTH- phenylephrine hcl tablet, coated
Nasal Decongestant PE by
Drug Labeling and Warnings
Nasal Decongestant PE by is a Otc medication manufactured, distributed, or labeled by Cardinal Health, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each tablet)
- Purpose
- Uses
-
Warnings
Do not use
if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- diabetes
- heart disease
- high blood pressure
- thyroid disease
- difficulty in urination due to enlargement of the prostate gland
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
LEADER™
NDC: 70000-0126-2
Maximum Strength | Non-Drowsy
Nasal
Decongestant PE
Phenylephrine HCl, 10 mg | Nasal DecongestantSinus Pressure + Congestion
72 TABLETS
COMPARE TO SUDAFED PE® CONGESTION MAXIMUM STRENGTH active ingredient*
100% Money Back GuaranteeTAMPER EVIDENT: DO NOT USE IF PACKAGE IS OPENED OR IF BLISTER UNIT IS TORN, BROKEN OR SHOWS ANY SIGNS OF TAMPERING
*This product is not manufactured or distributed by Johnson & Johnson Corporation, owner of the registered trademark Sudafed PE® Congestion Maximum Strength.
50844 REV0118A45323
DISTRIBUTED BY CARDINAL HEALTH
DUBLIN, OHIO 43017
www.myleader.com 1-800-200-6313
Essential to Care™ since 1979©2020 Cardinal Health. All Rights Reserved. CARDINAL HEALTH, the Cardinal Health LOGO, ESSENTIAL TO CARE, LEADER, and the Leader LOGO are trademarks or registered trademarks of Cardinal Health. All other marks are the property of their respective owners.
All LEADER™ Brand Products Have A
100% Money Back Guarantee
Return to place of purchase if not satisfied.
Leader 44-453
-
INGREDIENTS AND APPEARANCE
NASAL DECONGESTANT PE MAXIMUM STRENGTH
phenylephrine hcl tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 70000-0126 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PHENYLEPHRINE HYDROCHLORIDE (UNII: 04JA59TNSJ) (PHENYLEPHRINE - UNII:1WS297W6MV) PHENYLEPHRINE HYDROCHLORIDE 10 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DEXTROSE MONOHYDRATE (UNII: LX22YL083G) CALCIUM PHOSPHATE, DIBASIC, DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) CARBOXYMETHYLCELLULOSE SODIUM (UNII: K679OBS311) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) Product Characteristics Color RED Score no score Shape ROUND Size 7mm Flavor Imprint Code 44;453 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 70000-0126-1 1 in 1 CARTON 01/14/2005 1 18 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 70000-0126-2 3 in 1 CARTON 01/14/2005 2 24 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 01/14/2005 Labeler - Cardinal Health (097537435) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(70000-0126) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867894 MANUFACTURE(70000-0126) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(70000-0126) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(70000-0126) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(70000-0126)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.