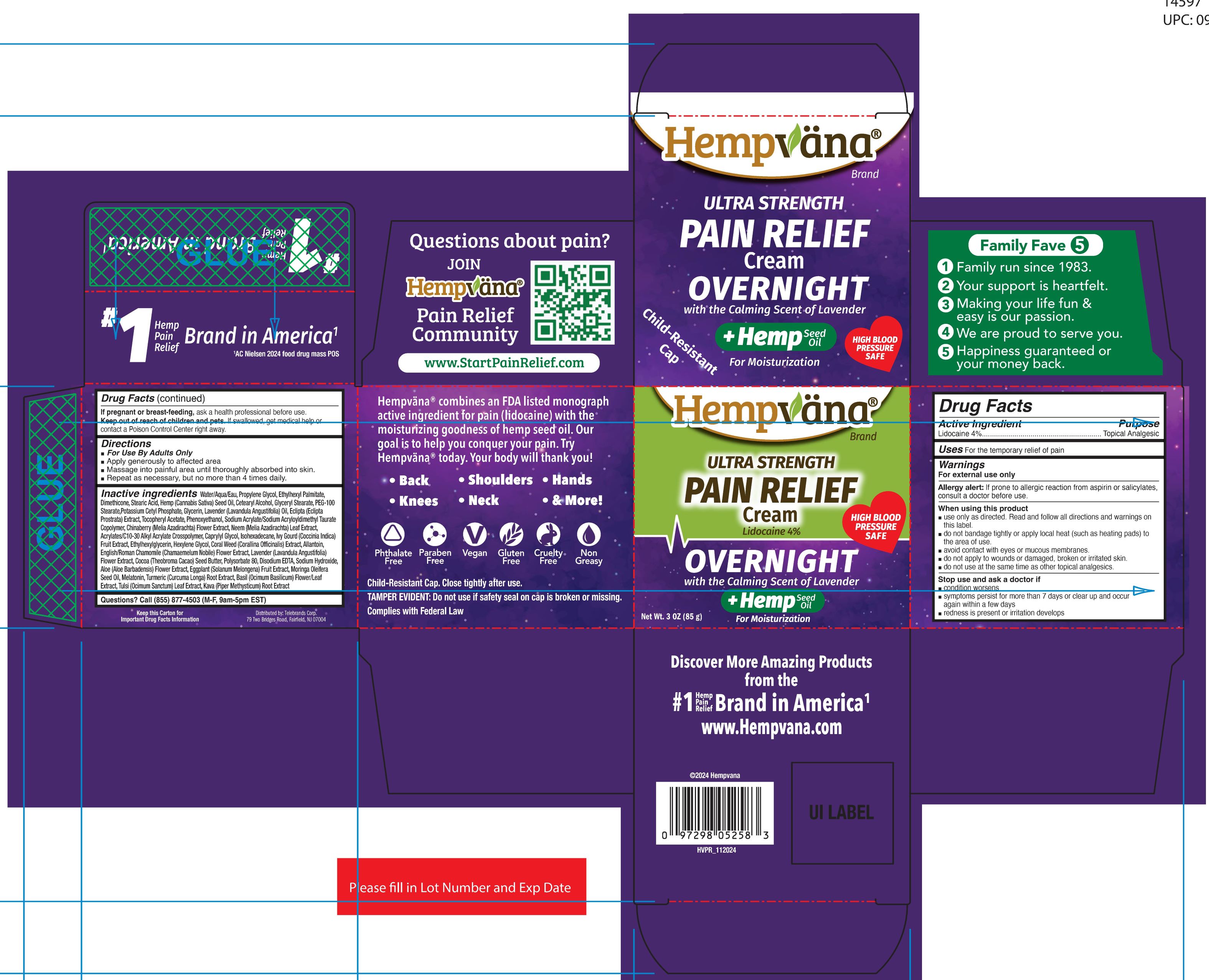
HEMPVANA ULTRA STRENGTH PAIN RELIEF - OVERNIGHT- lidocaine cream
Hempvana Ultra Strength Pain Relief - Overnight by
Drug Labeling and Warnings
Hempvana Ultra Strength Pain Relief - Overnight by is a Otc medication manufactured, distributed, or labeled by Telebrands Corp, Neutraderm, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert:If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
■ use only as directed. Read and follow all directions and warnings on this label.
■ do not bandage tightly or apply local heat (such as heating pads) to the area
of use.
■ avoid contact with eyes or mucous membranes.
■ do not apply to wounds or damaged, broken or irritated skin.
■ do not use at the same time as other topical analgesics.
■ do not use in large quantities, particularly over raw surfaces or blistered areas. - Directions
-
Inactive Ingredients
Water/Aqua/Eau, Propylene Glycol, Ethylhexyl Palmitate,Dimethicone, Stearic Acid, Hemp (Cannabis Sativa) Seed Oil, Cetearyl Alcohol, Glyceryl Stearate, PEG-100Stearate,Potassium Cetyl Phosphate, Glycerin, Lavender (Lavandula Angustifolia) Oil, Eclipta (EcliptaProstrata) Extract, Tocopheryl Acetate, Phenoxyethanol, Sodium Acrylate/Sodium Acryloyldimethyl TaurateCopolymer, Chinaberry (Melia Azadirachta) Flower Extract, Neem (Melia Azadirachta) Leaf Extract,Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Caprylyl Glycol, Isohexadecane, Ivy Gourd (Coccinia Indica)Fruit Extract, Ethylhexylglycerin, Hexylene Glycol, Coral Weed (Corallina Officinalis) Extract, Allantoin,English/Roman Chamomile (Chamaemelum Nobile) Flower Extract, Lavender (Lavandula Angustifolia)Flower Extract, Cocoa (Theobroma Cacao) Seed Butter, Polysorbate 80, Disodium EDTA, Sodium Hydroxide,Aloe (Aloe Barbadensis) Flower Extract, Eggplant (Solanum Melongena) Fruit Extract, Moringa OleiferaSeed Oil, Melatonin, Turmeric (Curcuma Longa) Root Extract, Basil (Ocimum Basilicum) Flower/LeafExtract, Tulsi (Ocimum Sanctum) Leaf Extract, Kava (Piper Methysticum) Root Extract
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMPVANA ULTRA STRENGTH PAIN RELIEF - OVERNIGHT
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73287-029 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength OCIMUM BASILICUM (BASIL) FLOWER/LEAF EXTRACT (UNII: 7SAB275FP2) ECLIPTA PROSTRATA WHOLE (UNII: 8138AB7E8Y) MELIA AZEDARACH WHOLE (UNII: 18X4G0SE73) ALOE BARBADENSIS FLOWER EXTRACT (UNII: 575DY8C1ER) CURCUMA LONGA (TURMERIC) ROOT EXTRACT (UNII: 856YO1Z64F) OCIMUM TENUIFLORUM WHOLE (UNII: 7OS52KZ33J) PEG-100 STEARATE (UNII: YD01N1999R) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) SODIUM HYDROXIDE (UNII: 55X04QC32I) STEARIC ACID (UNII: 4ELV7Z65AP) LAVANDULA ANGUSTIFOLIA FLOWER (UNII: 19AH1RAF4M) ETHYLHEXYL PALMITATE (UNII: 2865993309) WATER (UNII: 059QF0KO0R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) GLYCERIN (UNII: PDC6A3C0OX) ISOHEXADECANE (UNII: 918X1OUF1E) HEXYLENE GLYCOL (UNII: KEH0A3F75J) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) PHENOXYETHANOL (UNII: HIE492ZZ3T) DIMETHICONE (UNII: 92RU3N3Y1O) LAVENDER OIL (UNII: ZBP1YXW0H8) ALLANTOIN (UNII: 344S277G0Z) PIPER METHYSTICUM ROOT (UNII: BOW48C81XP) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) ALPHA-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCOA BUTTER (UNII: 512OYT1CRR) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) CHAMAEMELUM NOBILE FLOWER (UNII: O2T154T6OG) MELATONIN (UNII: JL5DK93RCL) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POLYSORBATE 80 (UNII: 6OZP39ZG8H) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) CORALLINA OFFICINALIS EXTRACT (UNII: 4004498D06) MELIA AZADIRACHTA LEAF (UNII: HKY915780T) COCCINIA INDICA FRUIT EXTRACT (UNII: VLJ6WOT3K5) MORINGA OLEIFERA SEED OIL (UNII: REM6A5QMC0) SOLANUM MELONGENA WHOLE (UNII: 5DS5EE0N93) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73287-029-01 1 in 1 CARTON 01/30/2024 1 85 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 01/30/2024 Labeler - Telebrands Corp (177266558) Establishment Name Address ID/FEI Business Operations Neutraderm, Inc. 146224444 manufacture(73287-029)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.