DUO FUSION- famotidine, calcium carbonate, magnesium hydroxide tablet, chewable
DUO Fusion by
Drug Labeling and Warnings
DUO Fusion by is a Otc medication manufactured, distributed, or labeled by Boehringer Ingelheim Pharmaceuticals, Inc., Perrigo. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- DOSAGE FORMS & STRENGTHS
- DOSAGE & ADMINISTRATION
- WHEN USING
- WARNINGS
- PURPOSE
- CLINICAL PHARMACOLOGY
- DO NOT USE
-
ASK DOCTOR
Ask a doctor before use if you have
- had heartburn over 3 months. This may be a sign of a more serious condition.
- heartburn with lightheadedness, sweating, or dizziness
- chest pain or shoulder pain with shortness of breath; sweating; pain spreading to arms, neck or shoulders; or lightheadedness
- frequent chest pain
- frequent wheezing, particularly with heartburn
- unexplained weight loss
- nausea or vomiting
- stomach pain
- QUESTIONS
- STOP USE
- PREGNANCY OR BREAST FEEDING
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- HOW SUPPLIED
-
INACTIVE INGREDIENT
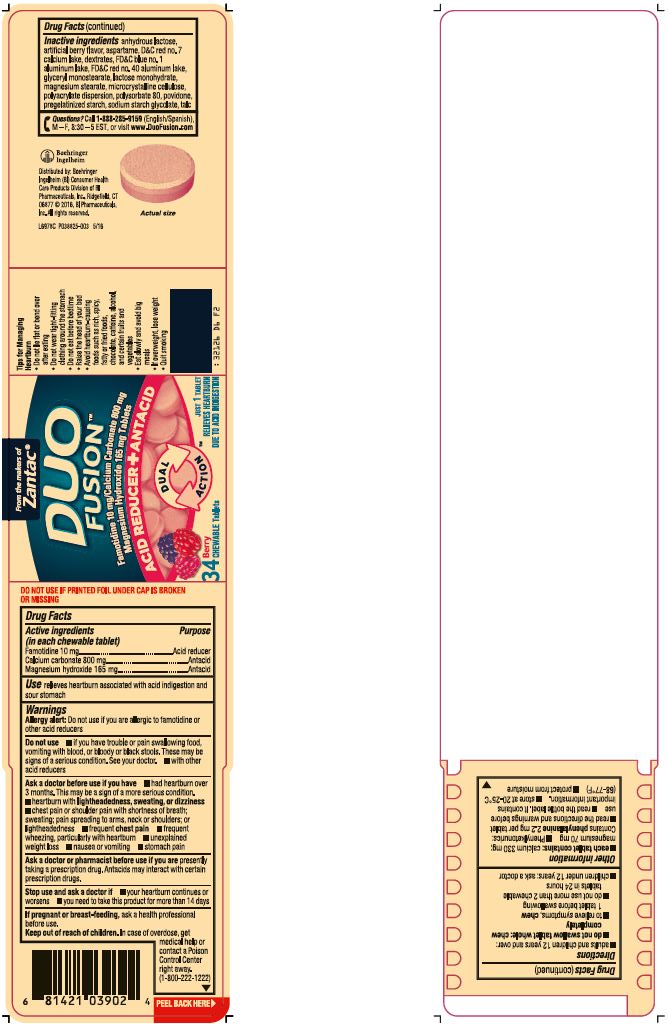
Inactive ingredients anhydrous lactose, artificial berry flavor, aspartame, D&C red no. 7 calcium lake, dextrates, FD&C blue no. 1 aluminum lake, FD&C red no. 40 aluminum lake, glycerol monostearate, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polyacrylate dispersion, polysorbate 80, povidone, pregelatinized starch, sodium starch glycolate, talc
- INFORMATION FOR PATIENTS
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DUO FUSION
famotidine, calcium carbonate, magnesium hydroxide tablet, chewableProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0597-0315 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Famotidine (UNII: 5QZO15J2Z8) (Famotidine - UNII:5QZO15J2Z8) Famotidine 10 mg Calcium Carbonate (UNII: H0G9379FGK) (CARBONATE ION - UNII:7UJQ5OPE7D) Calcium Carbonate 800 mg Magnesium Hydroxide (UNII: NBZ3QY004S) (Hydroxide ION - UNII:9159UV381P) Magnesium Hydroxide 165 mg Inactive Ingredients Ingredient Name Strength ANHYDROUS LACTOSE (UNII: 3SY5LH9PMK) ASPARTAME (UNII: Z0H242BBR1) D&C RED NO. 7 (UNII: ECW0LZ41X8) DEXTRATES (UNII: G263MI44RU) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) ALUMINUM OXIDE (UNII: LMI26O6933) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) METHACRYLIC ACID - ETHYL ACRYLATE COPOLYMER (1:1) TYPE A (UNII: NX76LV5T8J) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONES (UNII: FZ989GH94E) STARCH, CORN (UNII: O8232NY3SJ) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color PURPLE Score no score Shape ROUND Size 17mm Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0597-0315-97 20 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2016 2 NDC: 0597-0315-36 34 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2016 3 NDC: 0597-0315-08 55 in 1 BOTTLE; Type 0: Not a Combination Product 03/01/2016 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA077355 03/01/2016 Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) Establishment Name Address ID/FEI Business Operations Perrigo 006013346 PACK(0597-0315) , MANUFACTURE(0597-0315)
Trademark Results [DUO Fusion]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 DUO FUSION 97043914 not registered Live/Pending |
Nidec Motor Corporation 2021-09-24 |
 DUO FUSION 86748880 5005022 Live/Registered |
CHATTEM, INC. 2015-09-05 |
 DUO FUSION 75767230 2364499 Live/Registered |
ECOLAB USA INC. 1999-08-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.