Terazosin by Bionpharma Inc. / OrBion Pharmaceuticals Private Limited TERAZOSIN capsule
Terazosin by
Drug Labeling and Warnings
Terazosin by is a Prescription medication manufactured, distributed, or labeled by Bionpharma Inc., OrBion Pharmaceuticals Private Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Terazosin hydrochloride, USP an alpha-1-selective adrenoceptor blocking agent, is a quinazoline derivative represented by the following chemical name and structural formula: (RS)-Piperazine,1-(4-amino-6,7-dimethoxy-2-quinazolinyl)-4-[(tetra-hydro-2-furanyl)carbonyl]-, monohydrochloride, dihydrate.
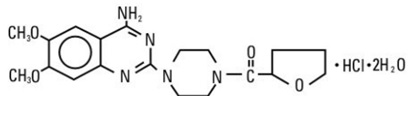
Terazosin hydrochloride, USP is a white to pale yellow crystalline powder, slightly soluble in water pH 1.3 (HCl), water pH 12.6 (NaOH), ethanol 95% and chloroform, sparingly soluble in water pH 6, soluble in methanol, and practically insoluble in n-hexane. Its molecular formula is C 19H 25N 5O 4HCl2H 2O and has a molecular weight of 459.93 g/mole.
Each capsule, for oral administration, contains terazosin hydrochloride, USP equivalent to 1 mg, 2 mg, 5 mg, or 10 mg of terazosin. In addition, each capsule contains the following inactive ingredients: Colloidal silicon dioxide, lactose monohydrate, magnesium stearate, and pregelatinized starch. The capsule shell contains D&C yellow no. 10 (2 mg, 5 mg and 10 mg), D&C red no. 28 (5 mg), FD& C green no. 3 (10 mg), FD&C red no. 40 (5 mg), gelatin, iron oxide black (1 mg), sodium lauryl sulfate, and titanium dioxide. The capsules are printed with black ink composed of ammonia solution, black iron oxide, potassium hydroxide, propylene glycol, and shellac.
FDA approved dissolution test specifications differ from USP.
-
CLINICAL PHARMACOLOGY
Pharmacodynamics
A. Benign Prostatic Hyperplasia (BPH)
The symptoms associated with BPH are related to bladder outlet obstruction, which is comprised of two underlying components: a static component and a dynamic component. The static component is a consequence of an increase in prostate size. Over time, the prostate will continue to enlarge. However, clinical studies have demonstrated that the size of the prostate does not correlate with the severity of BPH symptoms or the degree of urinary obstruction. The dynamic component is a function of an increase in smooth muscle tone in the prostate and bladder neck, leading to constriction of the bladder outlet. Smooth muscle tone is mediated by sympathetic nervous stimulation of alpha-1 adrenoceptors, which are abundant in the prostate, prostatic capsule and bladder neck. The reduction in symptoms and improvement in urine flow rates following administration of terazosin is related to relaxation of smooth muscle produced by blockade of alpha-1 adrenoceptors in the bladder neck and prostate. Because there are relatively few alpha-1 adrenoceptors in the bladder body, terazosin is able to reduce the bladder outlet obstruction without affecting bladder contractility.
Terazosin has been studied in 1,222 men with symptomatic BPH. In three placebo-controlled studies, symptom evaluation and uroflowmetric measurements were performed approximately 24 hours following dosing. Symptoms were quantified using the Boyarsky Index. The questionnaire evaluated both obstructive (hesitancy, intermittency, terminal dribbling, impairment of size and force of stream, sensation of incomplete bladder emptying) and irritative (nocturia, daytime frequency, urgency, dysuria) symptoms by rating each of the 9 symptoms from 0 to 3, for a total score of 27 points. Results from these studies indicated that terazosin statistically significantly improved symptoms and peak urine flow rates over placebo as follows:
Symptom Score
(Range 0 to 27)Peak Flow Rate
(mL/sec)N
Mean
Baseline
Change
Mean
(%)
N
Mean
Baseline
Change
Mean
(%)
Study 1 (10 mg) a
Titration to fixed dose (12 wks)
Placebo
Terazosin
55
54
9.7
10.1
-2.3
-4.5
(24)
(45) *
54
52
10.1
8.8
+1.0
+3.0
(10)
(34) *
Study 2 (2 mg, 5 mg, 10 mg, 20 mg) b
Titration to response (24 wks)
Placebo
Terazosin
89
85
12.5
12.2
-3.8
-5.3
(30)
(43) *
88
84
8.8
8.4
+1.4
+2.9
(16)
(35) *
Study 3 (1 mg, 2 mg, 5 mg, 10 mg) c
Titration to response (24 wks)
Placebo
Terazosin
74
73
10.4
10.9
-1.1
-4.6
(11)
(42) *
74
73
8.8
8.6
+1.2
+2.6
(14)
(30) *
aHighest dose 10 mg shown.
b23% of patients on 10 mg, 41% of patients on 20 mg.
c67% of patients on 10 mg.
*Significantly (p ≤ 0.05) more improvement than placebo.
In all three studies, both symptom scores and peak urine flow rates showed statistically significant improvement from baseline in patients treated with terazosin from week 2 (or the first clinic visit) and throughout the study duration.
Analysis of the effect of terazosin on individual urinary symptoms demonstrated that compared to placebo, terazosin significantly improved the symptoms of hesitancy, intermittency, impairment in size and force of urinary stream, sensation of incomplete emptying, terminal dribbling, daytime frequency and nocturia.
Global assessments of overall urinary function and symptoms were also performed by investigators who were blinded to patient treatment assignment. In studies 1 and 3, patients treated with terazosin had a significantly (p ≤ 0.001) greater overall improvement compared to placebo treated patients.
In a short-term study (study 1), patients were randomized to either 2 mg, 5 mg or 10 mg of terazosin or placebo. Patients randomized to the 10 mg group achieved a statistically significant response in both symptoms and peak flow rate compared to placebo (Figure 1).
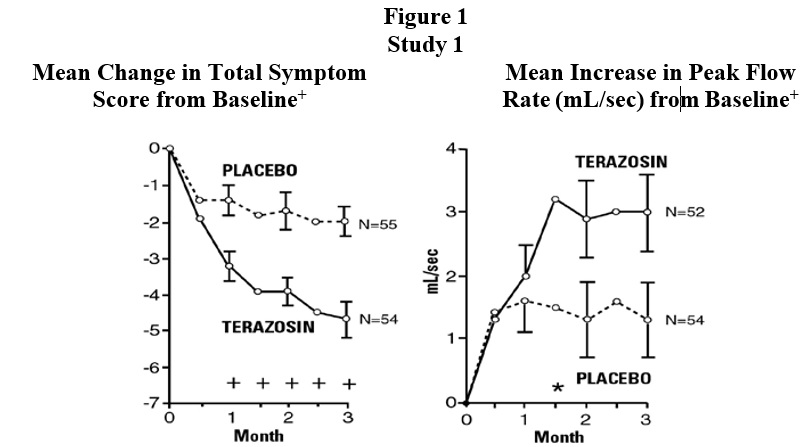
+ for baseline values see above table
* p ≤ 0.05, compared to placebo groupIn a long-term, open-label, non-placebo controlled clinical trial, 181 men were followed for 2 years and 58 of these men were followed for 30 months. The effect of terazosin on urinary symptom scores and peak flow rates was maintained throughout the study duration (Figures 2 and 3):
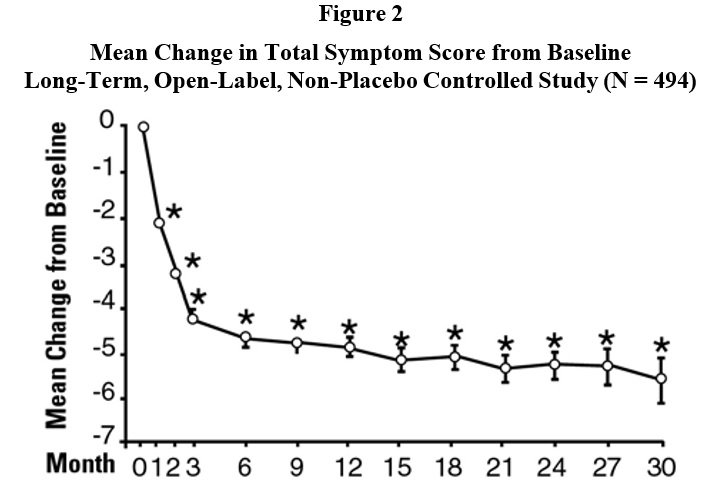
* p ≤ 0.05 vs. baseline
mean baseline = 10.7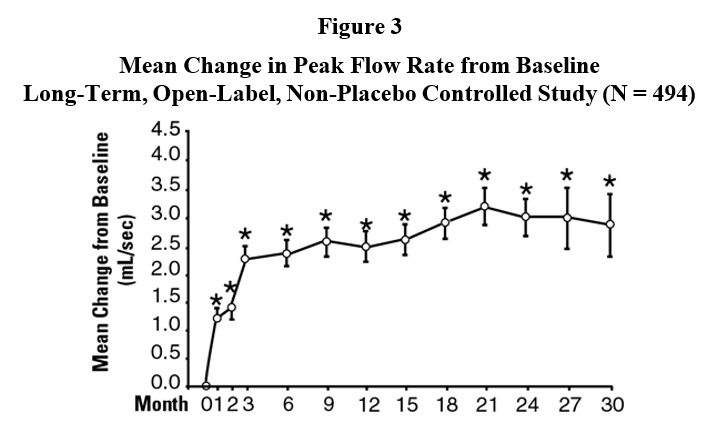
* p ≤ 0.05 vs. baseline
mean baseline = 9.9In this long-term trial, both symptom scores and peak urinary flow rates showed statistically significant improvement suggesting a relaxation of smooth muscle cells.
Although blockade of alpha-1 adrenoceptors also lowers blood pressure in hypertensive patients with increased peripheral vascular resistance, terazosin treatment of normotensive men with BPH did not result in a clinically significant blood pressure lowering effect:
Mean Changes in Blood Pressure from Baseline to Final Visit in all Double-Blind, Placebo-Controlled Studies
Normotensive Patients DBP ≤ 90 mmHg
Hypertensive Patients DBP > 90 mmHg
Group
N
Mean Change
N
Mean Change
SBP
(mmHg)Placebo
Terazosin293
519-0.1
-3.3 *45
65-5.8
-14.4 *DBP
(mmHg)Placebo
Terazosin293
519
+0.4
-2.2 *
45
65
-7.1
-15.1 *
*p ≤ 0.05 vs. placebo
B. Hypertension
In animals, terazosin causes a decrease in blood pressure by decreasing total peripheral vascular resistance. The vasodilatory hypotensive action of terazosin appears to be produced mainly by blockade of alpha-1 adrenoceptors. Terazosin decreases blood pressure gradually within 15 minutes following oral administration.
Patients in clinical trials of terazosin were administered once daily (the great majority) and twice daily regimens with total doses usually in the range of 5 mg/day to 20 mg/day, and had mild (about 77%, diastolic pressure 95 mmHg to 105 mmHg) or moderate (23%, diastolic pressure 105 mmHg to 115 mmHg) hypertension. Because terazosin, like all alpha antagonists, can cause unusually large falls in blood pressure after the first dose or first few doses, the initial dose was 1 mg in virtually all trials, with subsequent titration to a specified fixed dose or titration to some specified blood pressure end point (usually a supine diastolic pressure of 90 mmHg).
Blood pressure responses were measured at the end of the dosing interval (usually 24 hours) and effects were shown to persist throughout the interval, with the usual supine responses 5 mmHg to 10 mmHg systolic and 3.5 mmHg to 8 mmHg diastolic greater than placebo. The responses in the standing position tended to be somewhat larger, by 1 mmHg to 3 mmHg, although this was not true in all studies. The magnitude of the blood pressure responses was similar to prazosin and less than hydrochlorothiazide (in a single study of hypertensive patients). In measurements 24 hours after dosing, heart rate was unchanged.
Limited measurements of peak response (2 hours to 3 hours after dosing) during chronic terazosin administration indicate that it is greater than about twice the trough (24 hour) response, suggesting some attenuation of response at 24 hours, presumably due to a fall in blood terazosin concentrations at the end of the dose interval. This explanation is not established with certainty, however, and is not consistent with the similarity of blood pressure response to once daily and twice daily dosing and with the absence of an observed dose-response relationship over a range of 5 mg to 20 mg, i.e., if blood concentrations had fallen to the point of providing less than full effect at 24 hours, a shorter dosing interval or larger dose should have led to increased response.
Further dose response and dose duration studies are being carried out. Blood pressure should be measured at the end of the dose interval; if response is not satisfactory, patients may be tried on a larger dose or twice daily dosing regimen. The latter should also be considered if possibly blood pressure-related side effects, such as dizziness, palpitations, or orthostatic complaints, are seen within a few hours after dosing.
The greater blood pressure effect associated with peak plasma concentrations (first few hours after dosing) appears somewhat more position-dependent (greater in the erect position) than the effect of terazosin at 24 hours and in the erect position there is also a 6 beat to 10 beat per minute increase in heart rate in the first few hours after dosing. During the first 3 hours after dosing 12.5% of patients had a systolic pressure fall of 30 mmHg or more from supine to standing, or standing systolic pressure below 90 mmHg with a fall of at least 20 mmHg, compared to 4% of a placebo group.
There was a tendency for patients to gain weight during terazosin therapy. In placebo-controlled monotherapy trials, male and female patients receiving terazosin gained a mean of 1.7 pounds and 2.2 pounds respectively, compared to losses of 0.2 pound and 1.2 pounds respectively in the placebo group. Both differences were statistically significant.
During controlled clinical trials, patients receiving terazosin monotherapy had a small but statistically significant decrease (a 3% fall) compared to placebo in total cholesterol and the combined low-density and very-low-density lipoprotein fractions. No significant changes were observed in high-density lipoprotein fraction and triglycerides compared to placebo.
Analysis of clinical laboratory data following administration of terazosin suggested the possibility of hemodilution based on decreases in hematocrit, hemoglobin, white blood cells, total protein and albumin. Decreases in hematocrit and total protein have been observed with alpha-blockade and are attributed to hemodilution.
Pharmacokinetics
Terazosin hydrochloride administered as terazosin capsules is essentially completely absorbed in man. Administration of capsules immediately after meals had a minimal effect on the extent of absorption. The time to reach peak plasma concentration however, was delayed by about 40 minutes. Terazosin has been shown to undergo minimal hepatic first-pass metabolism and nearly all of the circulating dose is in the form of parent drug. The plasma levels peak about one hour after dosing, and then decline with a half-life of approximately 12 hours. In a study that evaluated the effect of age on terazosin pharmacokinetics, the mean plasma half-lives were 14 hours and 11.4 hours for the age group ≥ 70 years and the age group of 20 years to 39 years, respectively. After oral administration the plasma clearance was decreased by 31.7% in patients 70 years of age or older compared to that in patients 20 years to 39 years of age.
The drug is 90% to 94% bound to plasma proteins and binding is constant over the clinically observed concentration range. Approximately 10% of an orally administered dose is excreted as parent drug in the urine and approximately 20% is excreted in the feces. The remainder is eliminated as metabolites. Impaired renal function had no significant effect on the elimination of terazosin, and dosage adjustment of terazosin to compensate for the drug removal during hemodialysis (approximately 10%) does not appear to be necessary. Overall, approximately 40% of the administered dose is excreted in the urine and approximately 60% in the feces. The disposition of the compound in animals is qualitatively similar to that in man.
-
INDICATIONS AND USAGE
Terazosin capsules are indicated for the treatment of symptomatic benign prostatic hyperplasia (BPH). There is a rapid response, with approximately 70% of patients experiencing an increase in urinary flow and improvement in symptoms of BPH when treated with terazosin capsules. The long-term effects of terazosin on the incidence of surgery, acute urinary obstruction or other complications of BPH are yet to be determined.
Terazosin capsules are also indicated for the treatment of hypertension. It can be used alone or in combination with other antihypertensive agents such as diuretics or beta-adrenergic blocking agents.
- CONTRAINDICATIONS
-
WARNINGS
Syncope and "First-dose" Effect
Terazosin capsules, like other alpha-adrenergic blocking agents, can cause marked lowering of blood pressure, especially postural hypotension, and syncope in association with the first dose or first few days of therapy. A similar effect can be anticipated if therapy is interrupted for several days and then restarted. Syncope has also been reported with other alpha-adrenergic blocking agents in association with rapid dosage increases or the introduction of another antihypertensive drug. Syncope is believed to be due to an excessive postural hypotensive effect, although occasionally the syncopal episode has been preceded by a bout of severe supraventricular tachycardia with heart rates of 120 beats to 160 beats per minute.
Additionally, the possibility of the contribution of hemodilution to the symptoms of postural hypotension should be considered.
To decrease the likelihood of syncope or excessive hypotension, treatment should always be initiated with a 1 mg dose of terazosin, given at bedtime. The 2 mg, 5 mg and 10 mg capsules are not indicated as initial therapy. Dosage should then be increased slowly, according to recommendations in the Dosage and Administration section and additional antihypertensive agents should be added with caution. The patient should be cautioned to avoid situations, such as driving or hazardous tasks, where injury could result should syncope occur during initiation of therapy.
In early investigational studies, where increasing single doses up to 7.5 mg were given at 3 day intervals, tolerance to the first dose phenomenon did not necessarily develop and the "first dose" effect could be observed at all doses. Syncopal episodes occurred in 3 of the 14 subjects given terazosin at doses of 2.5 mg, 5 mg and 7.5 mg, which are higher than the recommended initial dose; in addition, severe orthostatic hypotension (blood pressure falling to 50/0 mmHg) was seen in two others and dizziness, tachycardia, and lightheadedness occurred in most subjects. These adverse effects all occurred within 90 minutes of dosing.
In three placebo-controlled BPH studies 1, 2, and 3 (see CLINICAL PHARMACOLOGY), the incidence of postural hypotension in the terazosin treated patients was 5.1%, 5.2%, and 3.7% respectively.
In multiple dose clinical trials involving nearly 2,000 hypertensive patients treated with terazosin, syncope was reported in about 1% of patients. Syncope was not necessarily associated only with the first dose.
If syncope occurs, the patient should be placed in a recumbent position and treated supportively as necessary. There is evidence that the orthostatic effect of terazosin is greater, even in chronic use, shortly after dosing. The risk of the events is greatest during the initial seven days of treatment, but continues at all time intervals.
Priapism
Rarely, (probably less than once in every several thousand patients) terazosin and other α 1-antagonists have been associated with priapism (painful penile erection, sustained for hours and unrelieved by sexual intercourse or masturbation). Two or three dozen cases have been reported. Because this condition can lead to permanent impotence if not promptly treated, patients must be advised about the seriousness of the condition (see PRECAUTIONS: Information for Patients).
-
PRECAUTIONS
General
Prostatic Cancer
Carcinoma of the prostate and BPH cause many of the same symptoms. These two diseases frequently co-exist. Therefore, patients thought to have BPH should be examined prior to starting terazosin therapy to rule out the presence of carcinoma of the prostate.
Intraoperative Floppy Iris Syndrome (IFIS)
Intraoperative Floppy Iris Syndrome (IFIS) has been observed during cataract surgery in some patients on/or previously treated with alpha-1 blockers. This variant of small pupil syndrome is characterized by the combination of a flaccid iris that billows in response to intraoperative irrigation currents, progressive intraoperative miosis despite preoperative dilation with standard mydriatic drugs, and potential prolapse of the iris toward the phacoemulsification incisions. The patient's ophthalmologist should be prepared for possible modifications to their surgical technique, such as the utilization of iris hooks, iris dilator rings, or viscoelastic substances. There does not appear to be a benefit of stopping alpha-1 blocker therapy prior to cataract surgery.
Orthostatic Hypotension
While syncope is the most severe orthostatic effect of terazosin (see WARNINGS), other symptoms of lowered blood pressure, such as dizziness, lightheadedness and palpitations, were more common and occurred in some 28% of patients in clinical trials of hypertension. In BPH clinical trials, 21% of the patients experienced one or more of the following: dizziness, hypotension, postural hypotension, syncope, and vertigo. Patients with occupations in which such events represent potential problems should be treated with particular caution.
Information for Patients(see Patient Package Insert)
Patients should be made aware of the possibility of syncopal and orthostatic symptoms, especially at the initiation of therapy, and to avoid driving or hazardous tasks for 12 hours after the first dose, after a dosage increase and after interruption of therapy when treatment is resumed. They should be cautioned to avoid situations where injury could result should syncope occur during initiation of terazosin therapy. They should also be advised of the need to sit or lie down when symptoms of lowered blood pressure occur, although these symptoms are not always orthostatic, and to be careful when rising from a sitting or lying position. If dizziness, lightheadedness, or palpitations are bothersome they should be reported to the physician, so that dose adjustment can be considered.
Patients should also be told that drowsiness or somnolence can occur with terazosin, requiring caution in people who must drive or operate heavy machinery.
Patients should be advised about the possibility of priapism as a result of treatment with terazosin and other similar medications. Patients should know that this reaction to terazosin is extremely rare, but that if it is not brought to immediate medical attention, it can lead to permanent erectile dysfunction (impotence).
Laboratory Tests
Small but statistically significant decreases in hematocrit, hemoglobin, white blood cells, total protein and albumin were observed in controlled clinical trials. These laboratory findings suggested the possibility of hemodilution. Treatment with terazosin for up to 24 months had no significant effect on prostate specific antigen (PSA) levels.
-
Drug Interactions
In controlled trials, terazosin has been added to diuretics, and several beta-adrenergic blockers; no unexpected interactions were observed. Terazosin has also been used in patients on a variety of concomitant therapies; while these were not formal interaction studies, no interactions were observed. Terazosin has been used concomitantly in at least 50 patients on the following drugs or drug classes:
1) analgesic/anti-inflammatory (e.g., acetaminophen, aspirin, codeine, ibuprofen, indomethacin).
2) antibiotics (e.g., erythromycin, trimethoprim and sulfamethoxazole).
3) anticholinergic/sympathomimetics (e.g., phenylephrine hydrochloride, phenylpropanolamine hydrochloride, pseudoephedrine hydrochloride).
4) antigout (e.g., allopurinol).
5) antihistamines (e.g., chlorpheniramine).
6) cardiovascular agents (e.g., atenolol, hydrochlorothiazide, methyclothiazide, propranolol).
7) corticosteroids.
8) gastrointestinal agents (e.g., antacids).
9) hypoglycemics.
10) sedatives and tranquilizers (e.g., diazepam).
Use with Other Drugs
In a study (n = 24) where terazosin and verapamil were administered concomitantly, terazosin's mean AUC 0-24increased 11% after the first verapamil dose and after 3 weeks of verapamil treatment it increased by 24% with associated increases in C max(25%) and C min(32%) means. Terazosin mean T maxdecreased from 1.3 hours to 0.8 hours after 3 weeks of verapamil treatment.
Statistically significant differences were not found in the verapamil level with and without terazosin. In a study (n = 6) where terazosin and captopril were administered concomitantly, plasma disposition of captopril was not influenced by concomitant administration of terazosin and terazosin maximum plasma concentrations increased linearly with dose at steady-state after administration of terazosin plus captopril (see DOSAGE AND ADMINISTRATION).
Carcinogenesis, Mutagenesis, Impairment of Fertility
Terazosin was devoid of mutagenic potential when evaluated in vivoand in vitro(the Ames test, in vivo cytogenetics, the dominant lethal test in mice, in vivoChinese hamster chromosome aberration test and V79 forward mutation assay).
Terazosin, administered in the feed to rats at doses of 8 mg/kg/day, 40 mg/kg/day, and 250 mg/kg/day (70 mg/m 2/day, 350 mg/m 2/day, and 2,100 mg/m 2/day), for two years, was associated with a statistically significant increase in benign adrenal medullary tumors of male rats exposed to the 250 mg/kg dose. This dose is 175 times the maximum recommended human dose of 20 mg (12 mg/m 2). Female rats were unaffected. Terazosin was not oncogenic in mice when administered in feed for 2 years at a maximum tolerated dose of 32 mg/kg/day (110 mg/m 2; 9 times the maximum recommended human dose). The absence of mutagenicity in a battery of tests, of tumorigenicity of any cell type in the mouse carcinogenicity assay, of increased total tumor incidence in either species, and of proliferative adrenal lesions in female rats, suggests a male rat species-specific event. Numerous other diverse pharmaceutical and chemical compounds have also been associated with benign adrenal medullary tumors in male rats without supporting evidence for carcinogenicity in man.
The effect of terazosin on fertility was assessed in a standard fertility/reproductive performance study in which male and female rats were administered oral doses of 8 mg/kg/day, 30 mg/kg/day and 120 mg/kg/day. Four of 20 male rats given 30 mg/kg (240 mg/m 2; 20 times the maximum recommended human dose) and five of 19 male rats given 120 mg/kg (960 mg/m 2; 80 times the maximum recommended human dose) failed to sire a litter. Testicular weights and morphology were unaffected by treatment. Vaginal smears at 30 mg/kg/day and 120 mg/kg/day, however, appeared to contain less sperm than smears from control matings and good correlation was reported between sperm count and subsequent pregnancy.
Oral administration of terazosin for one or two years elicited a statistically significant increase in the incidence of testicular atrophy in rats exposed to 40 mg/kg/day and 250 mg/kg/day (29 times and 175 times the maximum recommended human dose), but not in rats exposed to 8 mg/kg/day (> 6 times the maximum recommended human dose). Testicular atrophy was also observed in dogs dosed with 300 mg/kg/day (> 500 times the maximum recommended human dose) for three months but not after one year when dosed with 20 mg/kg/day (38 times the maximum recommended human dose). This lesion has also been seen with prazosin hydrochloride, another (marketed) selective-alpha-1 blocking agent.
-
Pregnancy
Teratogenic effects: Terazosin was not teratogenic in either rats or rabbits when administered at oral doses up to 280 times and 60 times, respectively, the maximum recommended human dose. Fetal resorptions occurred in rats dosed with 480 mg/kg/day, approximately 280 times the maximum recommended human dose. Increased fetal resorptions, decreased fetal weight and an increased number of supernumerary ribs were observed in offspring of rabbits dosed with 60 times the maximum recommended human dose. These findings (in both species) were most likely secondary to maternal toxicity. There are no adequate and well-controlled studies in pregnant women and the safety of terazosin in pregnancy has not been established. Terazosin is not recommended during pregnancy unless the potential benefit justifies the potential risk to the mother and fetus.
Nonteratogenic effects: In a peri- and post-natal development study in rats, significantly more pups died in the group dosed with 120 mg/kg/day (> 75 times the maximum recommended human dose) than in the control group during the three-week postpartum period.
Nursing Mothers
It is not known whether terazosin is excreted in breast milk. Because many drugs are excreted in breast milk, caution should be exercised when terazosin is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in children have not been determined.
-
ADVERSE REACTIONS
Benign Prostatic Hyperplasia
The incidence of treatment-emergent adverse events has been ascertained from clinical trials conducted worldwide. All adverse events reported during these trials were recorded as adverse reactions. The incidence rates presented below are based on combined data from six placebo-controlled trials involving once-a-day administration of terazosin at doses ranging from 1 mg to 20 mg. Table 1 summarizes those adverse events reported for patients in these trials when the incidence rate in the terazosin group was at least 1% and was greater than that for the placebo group, or where the reaction is of clinical interest. Asthenia, postural hypotension, dizziness, somnolence, nasal congestion/rhinitis, and impotence were the only events that were significantly (p ≤ 0.05) more common in patients receiving terazosin than in patients receiving placebo. The incidence of urinary tract infection was significantly lower in the patients receiving terazosin than in patients receiving placebo. An analysis of the incidence rate of hypotensive adverse events (see PRECAUTIONS) adjusted for the length of drug treatment has shown that the risk of the events is greatest during the initial seven days of treatment, but continues at all time intervals.
TABLE 1: ADVERSE REACTIONS DURING PLACEBO-CONTROLLED TRIALS BENIGN PROSTATIC HYPERPLASIA
BODY SYSTEM
TERAZOSIN
(N = 636)
PLACEBO
(N = 360)
BODY AS A WHOLE
†Asthenia
7.4% *
3.3%
Flu Syndrome
2.4%
1.7%
Headache
4.9%
5.8%
CARDIOVASCULAR SYSTEM
Hypotension
0.6%
0.6%
Palpitations
0.9%
1.1%
Postural Hypotension
3.9% *
0.8%
Syncope
0.6%
0.0%
DIGESTIVE SYSTEM
Nausea
1.7%
1.1%
METABOLIC AND NUTRITIONAL DISORDERS
Peripheral Edema
0.9%
0.3%
Weight Gain
0.5%
0.0%
NERVOUS SYSTEM
Dizziness
9.1% *
4.2%
Somnolence
3.6% *
1.9%
Vertigo
1.4%
0.3%
RESPIRATORY SYSTEM
Dyspnea
1.7%
0.8%
Nasal Congestion/Rhinitis
1.9% *
0.0%
SPECIAL SENSES
Blurred Vision/Amblyopia
1.3%
0.6%
UROGENITAL SYSTEM
Impotence
1.6% *
0.6%
Urinary Tract Infection
1.3%
3.9% *
† Includes weakness, tiredness, lassitude and fatigue.
* p ≤ 0.05 comparison between groups.
Additional adverse events have been reported, but these are, in general, not distinguishable from symptoms that might have occurred in the absence of exposure to terazosin. The safety profile of patients treated in the long-term open-label study was similar to that observed in the controlled studies.
The adverse events were usually transient and mild or moderate in intensity, but sometimes were serious enough to interrupt treatment. In the placebo-controlled clinical trials, the rates of premature termination due to adverse events were not statistically different between the placebo and terazosin groups. The adverse events that were bothersome, as judged by their being reported as reasons for discontinuation of therapy by at least 0.5% of the terazosin group and being reported more often than in the placebo group, are shown in Table 2.
TABLE 2: DISCONTINUATION DURING PLACEBO-CONTROLLED TRIALS BENIGN PROSTATIC HYPERPLASIA
BODY SYSTEM
TERAZOSIN
(N = 636)PLACEBO
(N = 360)BODY AS A WHOLE
Fever
0.5%
0.0%
Headache
1.1%
0.8%
CARDIOVASCULAR SYSTEM
Postural Hypotension
0.5%
0.0%
Syncope
0.5%
0.0%
DIGESTIVE SYSTEM
Nausea
0.5%
0.3%
NERVOUS SYSTEM
Dizziness
2.0%
1.1%
Vertigo
0.5%
0.0%
RESPIRATORY SYSTEM
Dyspnea
0.5%
0.3%
SPECIAL SENSES
Blurred Vision/Amblyopia
0.6%
0.0%
UROGENITAL SYSTEM
Urinary Tract Infection
0.5%
0.3%
Hypertension
The prevalence of adverse reactions has been ascertained from clinical trials conducted primarily in the United States. All adverse experiences (events) reported during these trials were recorded as adverse reactions. The prevalence rates presented below are based on combined data from fourteen placebo-controlled trials involving once-a-day administration of terazosin, as monotherapy or in combination with other antihypertensive agents, at doses ranging from 1 mg to 40 mg. Table 3 summarizes those adverse experiences reported for patients in these trials where the prevalence rate in the terazosin group was at least 5%, where the prevalence rate for the terazosin group was at least 2% and was greater than the prevalence rate for the placebo group, or where the reaction is of particular interest. Asthenia, blurred vision, dizziness, nasal congestion, nausea, peripheral edema, palpitations and somnolence were the only symptoms that were significantly (p < 0.05) more common in patients receiving terazosin than in patients receiving placebo. Similar adverse reaction rates were observed in placebo-controlled monotherapy trials.
TABLE 3: ADVERSE REACTIONS DURING PLACEBO-CONTROLLED TRIALS HYPERTENSION
Body System Terazosin
(N = 859)Placebo
(N = 506)BODY AS A WHOLE †Asthenia 11.3%* 4.3% Back Pain 2.4% 1.2% Headache 16.2% 15.8% CARDIOVASCULAR SYSTEM Palpitations 4.3%* 1.2% Postural Hypotension 1.3% 0.4% Tachycardia 1.9% 1.2% DIGESTIVE SYSTEM Nausea 4.4%* 1.4% METABOLIC AND NUTRITIONAL DISORDERS Edema 0.9% 0.6% Peripheral Edema 5.5%* 2.4% Weight Gain 0.5% 0.2% MUSCULOSKELETAL SYSTEM Pain-Extremities 3.5% 3.0% NERVOUS SYSTEM Depression 0.3% 0.2% Dizziness 19.3%* 7.5% Libido Decreased 0.6% 0.2% Nervousness 2.3% 1.8% Paresthesia 2.9% 1.4% Somnolence 5.4%* 2.6% RESPIRATORY SYSTEM Dyspnea 3.1% 2.4% Nasal Congestion 5.9%* 3.4% Sinusitis 2.6% 1.4% SPECIAL SENSES Blurred Vision 1.6%* 0.0% UROGENITAL SYSTEM Impotence 1.2% 1.4% * Includes weakness, tiredness, lassitude, and fatigue.
† Statistically significant at p = 0.05 level.
Additional adverse reactions have been reported, but these are, in general, not distinguishable from symptoms that might have occurred in the absence of exposure to terazosin. The following additional adverse reactions were reported by at least 1% of 1,987 patients who received terazosin in controlled or open, short- or long-term clinical trials or have been reported during marketing experience:
Body as a Whole:chest pain, facial edema, fever, abdominal pain, neck pain, shoulder pain.
Cardiovascular System:arrhythmia, vasodilation.
Digestive System:constipation, diarrhea, dry mouth, dyspepsia, flatulence, vomiting.
Metabolic/Nutritional Disorders:gout.
Musculoskeletal System:arthralgia, arthritis, joint disorder, myalgia.
Nervous System:anxiety, insomnia.
Respiratory System:bronchitis, cold symptoms, epistaxis, flu symptoms, increased cough, pharyngitis, rhinitis.
Skin and Appendages:pruritus, rash, sweating.
Special Senses:abnormal vision, conjunctivitis, tinnitus.
Urogenital System:urinary frequency, urinary incontinence primarily reported in postmenopausal women, urinary tract infection.
The adverse reactions were usually mild or moderate in intensity but sometimes were serious enough to interrupt treatment. The adverse reactions that were most bothersome, as judged by their being reported as reasons for discontinuation of therapy by at least 0.5% of the terazosin group and being reported more often than in the placebo group, are shown in Table 4.
TABLE 4: DISCONTINUATIONS DURING PLACEBO-CONTROLLED TRIALS HYPERTENSION
Body System Terazosin
(N = 859)Placebo
(N = 506)BODY AS A WHOLE Asthenia 1.6% 0.0% Headache 1.3% 1.0% CARDIOVASCULAR SYSTEM Palpitations 1.4% 0.2% Postural Hypotension 0.5% 0.0% Syncope 0.5% 0.2% Tachycardia 0.6% 0.0% DIGESTIVE SYSTEM Nausea 0.8% 0.0% METABOLIC AND NUTRITIONAL DISORDERS Peripheral Edema 0.6% 0.0% NERVOUS SYSTEM Dizziness 3.1% 0.4% Paresthesia 0.8% 0.2% Somnolence 0.6% 0.2% RESPIRATORY SYSTEM Dyspnea 0.9% 0.6% Nasal Congestion 0.6% 0.0% SPECIAL SENSES Blurred Vision 0.6% 0.0% Post-marketing Experience
Post-marketing experience indicates that in rare instances patients may develop allergic reactions, including anaphylaxis, following administration of terazosin hydrochloride. There have been reports of priapism and thrombocytopenia during post-marketing surveillance. Atrial fibrillation has been reported.
During cataract surgery, a variant of small pupil syndrome known as Intraoperative Floppy Iris Syndrome (IFIS) has been reported in association with alpha-1 blocker therapy (see PRECAUTIONS).
To report SUSPECTED ADVERSE REACTIONS, contact Chartwell RX, LLC. at 1-845-232-1683 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
-
OVERDOSAGE
Should overdosage of terazosin lead to hypotension, support of the cardiovascular system is of first importance. Restoration of blood pressure and normalization of heart rate may be accomplished by keeping the patient in the supine position. If this measure is inadequate, shock should first be treated with volume expanders. If necessary, vasopressors should then be used and renal function should be monitored and supported as needed. Laboratory data indicate that terazosin is 90% to 94% protein bound; therefore, dialysis may not be of benefit.
-
DOSAGE AND ADMINISTRATION
If terazosin capsules administration is discontinued for several days, therapy should be reinstituted using the initial dosing regimen.
Benign Prostatic Hyperplasia
Initial Dose
1 mg at bedtime is the starting dose for all patients, and this dose should not be exceeded as an initial dose. Patients should be closely followed during initial administration in order to minimize the risk of severe hypotensive response.
Subsequent Doses
The dose should be increased in a stepwise fashion to 2 mg, 5 mg, or 10 mg once daily to achieve the desired improvement of symptoms and/or flow rates. Doses of 10 mg once daily are generally required for the clinical response. Therefore, treatment with 10 mg for a minimum of 4 weeks to 6 weeks may be required to assess whether a beneficial response has been achieved. Some patients may not achieve a clinical response despite appropriate titration. Although some additional patients responded at a 20 mg daily dose, there was an insufficient number of patients studied to draw definitive conclusions about this dose. There are insufficient data to support the use of higher doses for those patients who show inadequate or no response to 20 mg daily. If terazosin administration is discontinued for several days or longer, therapy should be reinstituted using the initial dosing regimen.
Use with Other Drugs
Caution should be observed when terazosin capsules are administered concomitantly with other antihypertensive agents, especially the calcium channel blocker verapamil, to avoid the possibility of developing significant hypotension. When using terazosin and other antihypertensive agents concomitantly, dosage reduction and retitration of either agent may be necessary (see PRECAUTIONS).
Hypotension has been reported when terazosin capsules have been used with phosphodiesterase-5 (PDE-5) inhibitors.
Hypertension
The dose of terazosin capsules and the dose interval (12 hours or 24 hours) should be adjusted according to the patient's individual blood pressure response. The following is a guide to its administration:
Initial Dose
1 mg at bedtime is the starting dose for all patients, and this dose should not be exceeded. This initial dosing regimen should be strictly observed to minimize the potential for severe hypotensive effects.
Subsequent Doses
The dose may be slowly increased to achieve the desired blood pressure response. The usual recommended dose range is 1 mg to 5 mg administered once a day; however, some patients may benefit from doses as high as 20 mg per day. Doses over 20 mg do not appear to provide further blood pressure effect and doses over 40 mg have not been studied. Blood pressure should be monitored at the end of the dosing interval to be sure control is maintained throughout the interval. It may also be helpful to measure blood pressure 2 hours to 3 hours after dosing to see if the maximum and minimum responses are similar, and to evaluate symptoms such as dizziness or palpitations which can result from excessive hypotensive response. If response is substantially diminished at 24 hours an increased dose or use of a twice daily regimen can be considered. If terazosin administration is discontinued for several days or longer, therapy should be reinstituted using the initial dosing regimen.
In clinical trials, except for the initial dose, the dose was given in the morning.
Use with other drugs: (see above)
-
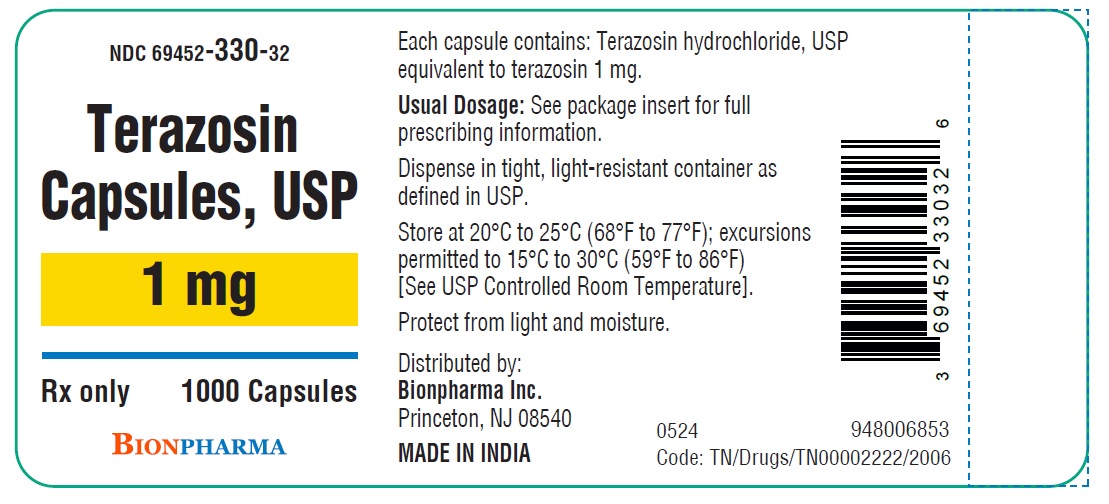
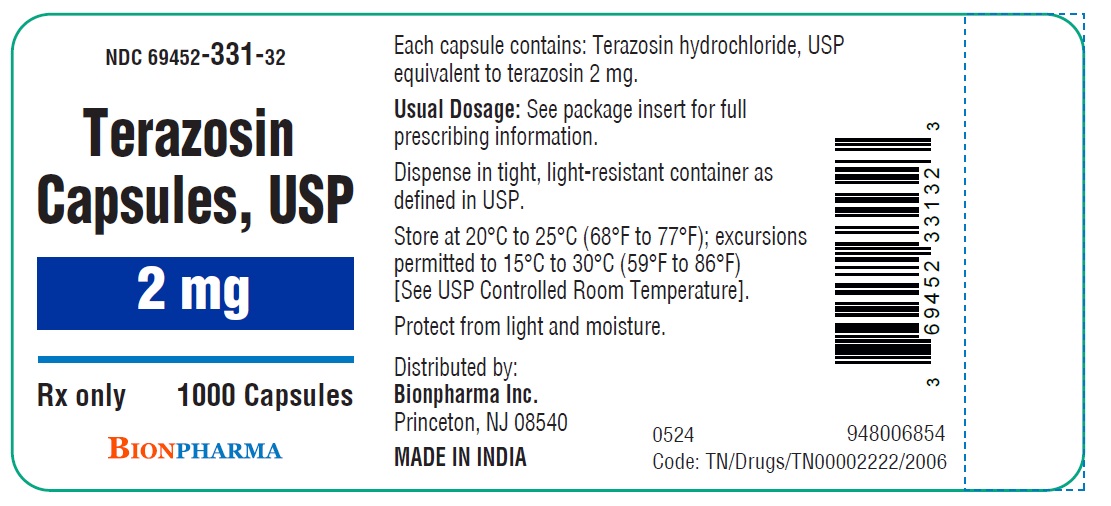
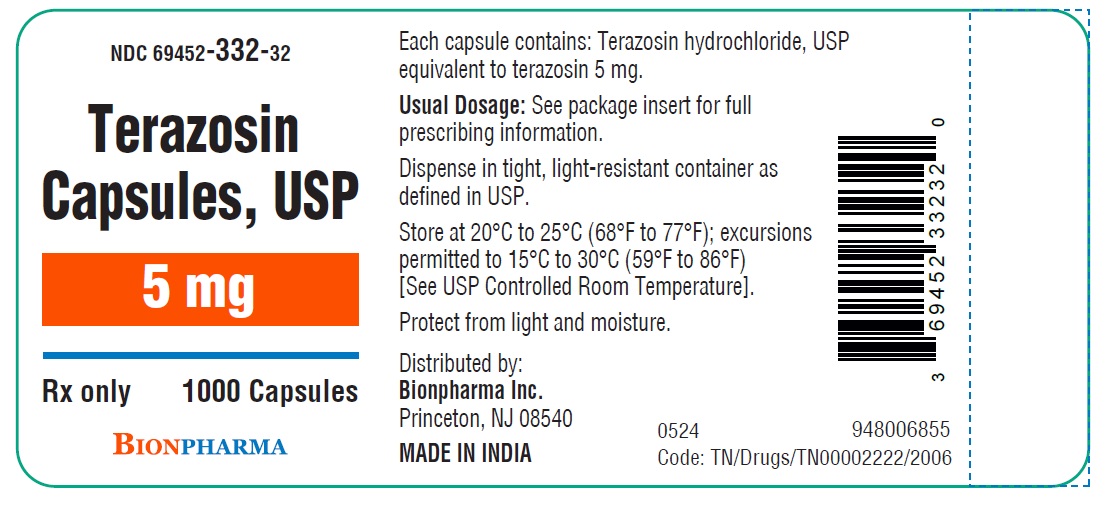
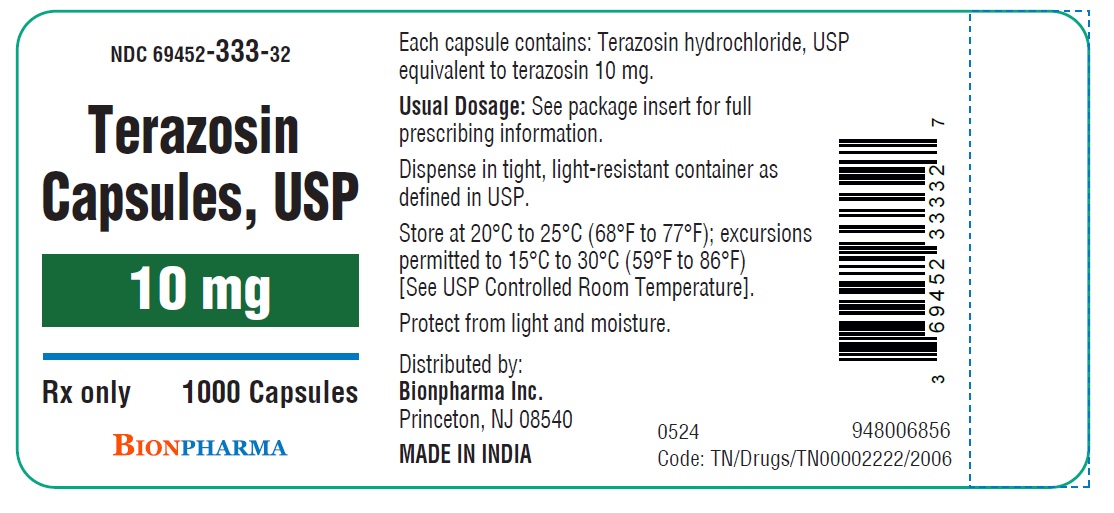
HOW SUPPLIED
Terazosin capsules, USP are available in four dosage strengths
1 mg gray capsules available as hard gelatin capsule with gray opaque cap and gray opaque body filled with white to off white powder blend. The capsule is printed with “ TE1” in black ink on the body, free from physical defects.
Bottles of 100....................................................( NDC 69452-330-20)
Bottles of 1000..................................................( NDC 69452-330-32)
2 mg yellow capsules available as hard gelatin capsule with ivory opaque cap and ivory opaque body filled with white to off white powder blend. The capsule is printed with “ TE2” in black ink on the body, free from physical defects.
Bottles of 100....................................................( NDC 69452-331-20)
Bottles of 1000..................................................( NDC 69452-331-32)
5 mg red capsules available as hard gelatin capsule with orange opaque cap and orange opaque body filled with white to off white powder blend. The capsule is printed with “ TE3” in black ink on the body, free from physical defects.
Bottles of 100....................................................( NDC 69452-332-20)
Bottles of 1000..................................................( NDC 69452-332-32)
10 mg blue capsules available as hard gelatin capsule with green opaque cap and green opaque body filled with white to off white powder blend. The capsule is printed with “ TE4” in black ink on the body, free from physical defects.
Bottles of 100....................................................( NDC 69452-333-20)
Bottles of 1000..................................................( NDC 69452-333-32)
Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [See USP Controlled Room Temperature]. Protect from light and moisture.
Dispense in tight, light-resistant container as defined in USP.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
MADE IN INDIA
Revised: 05/24
FDA-03
-
PATIENT INFORMATION ABOUT
Terazosin (ter-A-zo-sin)
Capsules, USPWhen used to treat HYPERTENSION or BENIGN PROSTATIC HYPERPLASIA (BPH)
Please read this leaflet before you start taking terazosin capsules. Also, read it each time you get a new prescription. This is a summary and should NOT take the place of a full discussion with your doctor who has additional information about terazosin capsules. You and your doctor should discuss terazosin capsules and your condition before you start taking it and at your regular check-ups.
Terazosin capsules are used to treat high blood pressure (hypertension). Terazosin capsules are also used to treat benign prostatic hyperplasia (BPH) in men. This leaflet describes terazosin capsules as a treatment for hypertension or BPH.
What is hypertension (high blood pressure)?
Blood pressure is the tension of the blood within the blood vessels. If blood is pumped too forcefully, or if the blood vessels are too narrow, the pressure of the blood against the walls of the vessels rises.
If high blood pressure is not treated, over time, the increased pressure can damage blood vessels or it can cause the heart to work too hard and may decrease the flow of blood to the heart, brain, and kidneys. As a result, these organs may become damaged and not function correctly. If high blood pressure is controlled, this damage is less likely to happen.
Treatment options for hypertension
Non-drug treatments are sometimes effective in controlling mild hypertension. The most important lifestyle changes to lower blood pressure are to lose weight, reduce salt, fat, and alcohol in the diet, quit smoking, and exercise regularly. However, many hypertensive patients require one or more ongoing medications to control their blood pressure. There are different kinds of medications used to treat hypertension. Your doctor has prescribed terazosin capsules for you.
What terazosin capsules do to treat hypertension?
Terazosin capsules work by relaxing blood vessels so that blood passes through them more easily. This helps to lower blood pressure.
What is BPH?
The prostate is a gland located below the bladder of men. It surrounds the urethra (you-REETH-rah), which is a tube that drains urine from the bladder. BPH is an enlargement of the prostate gland. The symptoms of BPH, however, can be caused by an increase in the tightness of muscles in the prostate. If the muscles inside the prostate tighten, they can squeeze the urethra and slow the flow of urine. This can lead to symptoms such as:
- a weak or interrupted stream when urinating
- a feeling that you cannot empty your bladder completely
- a feeling of delay when you start to urinate
- a need to urinate often, especially at night, or
- a feeling that you must urinate right away.
Treatment options for BPH
There are three main treatment options for BPH:
- Program of monitoring or "Watchful Waiting".Some men have an enlarged prostate gland, but no symptoms, or symptoms that are not bothersome. If this applies, you and your doctor may decide on a program of monitoring including regular checkups, instead of medication or surgery.
- Medication.There are different kinds of medication used to treat BPH. Your doctor has prescribed terazosin capsules for you. See "What terazosin capsules do to treat BPH"below.
- Surgery.Some patients may need surgery. Your doctor can describe several different surgical procedures to treat BPH. Which procedure is best depends on your symptoms and medical condition.
What terazosin capsules do to treat BPH?
Terazosin capsules relax the tightness of a certain type of muscle in the prostate and at the opening of the bladder. This may increase the rate of urine flow and/or decrease the symptoms you are having.
- Terazosin capsules help elieve the symptoms of BPH. It does NOT change the size of the prostate, which may continue to grow. However, a larger prostate does not necessarily cause more or worse symptoms.
- If terazosin capsules are helping you, you should notice an effect on your particular symptoms in 2 weeks to 4 weeks of starting to take the medication.
- Even though you take terazosin capsules and it may help you, terazosin capsules may not prevent the need for surgery in the future.
Other important facts about terazosin capsules for BPH
- You should see an effect on your symptoms in 2 weeks to 4 weeks. So, you will need to continue seeing your doctor to check your progress regarding your BPH and to monitor your blood pressure in addition to your other regular check-ups.
- Your doctor has prescribed terazosin capsules for your BPH and not for prostate cancer. However, a man can have BPH and prostate cancer at the same time. Doctors usually recommend that men be checked for prostate cancer once a year when they turn 50 (or 40 if a family member has had prostate cancer). These checks should continue even if you are taking terazosin capsules. Terazosin capsules are not a treatment for prostate cancer.
- About Prostate Specific Antigen (PSA). Your doctor may have done a blood test called PSA. Your doctor is aware that terazosin capsules do not affect PSA levels. You may want to ask your doctor more about this if you have had a PSA test done.
What you should know while taking terazosin capsules for hypertension or BPH?
WARNINGS
Terazosin Capsules Can Cause a Sudden Drop in Blood Pressure After the VERY FIRST DOSE.You may feel dizzy, faint, or "light-headed" particularly after you get up from bed or from a chair. This is more likely to occur after you've taken the first few doses, but can occur at any time while you are taking the drug. It can also occur if you stop taking the drug and then re-start treatment.
Because of this effect, your doctor may have told you to take terazosin capsules at bedtime. If you take terazosin capsules at bedtime but need to get up from bed to go to the bathroom, get up slowly and cautiously until you are sure how the medicine affects you. It is also important to get up slowly from a chair or bed at any time until you learn how you react to terazosin capsules. You should not drive or do any hazardous tasks until you are used to the effects of the medication. If you begin to feel dizzy, sit or lie down until you feel better.
- You will start with a 1 mg dose of terazosin capsules. Then the dose will be increased as your body gets used to the effect of the medication.
- Other side effects you could have while taking terazosin capsules include drowsiness, blurred or hazy vision, nausea, or "puffiness" of the feet or hands. Discuss any unexpected effects you notice with your doctor.
Extremely rarely, terazosin capsules and similar medications have caused painful erection of the penis, sustained for hours and unrelieved by sexual intercourse or masturbation. This condition is serious, and if untreated it can be followed by permanent inability to have an erection. If you have a prolonged abnormal erection, call your doctor or go to an emergency room as soon as possible.
Call you doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How to take terazosin capsules?
Follow your doctor's instructions about how to take terazosin capsules. You must take it every day at the dose prescribed. Talk with your doctor if you don't take it for a few days, you may have to restart it at a 1 mg dose and be cautious about possible dizziness. Do not share terazosin capsules with anyone else; it was prescribed only for you.
Keep terazosin capsules and all medicines out of the reach of children.
Store capsules between 68°F to 77°F (20°C to 25°C). Protect from light and moisture.
FOR MORE INFORMATION ABOUT TERAZOSIN CAPSULES AND HYPERTENSION OR BPH, TALK WITH YOUR DOCTOR, NURSE, PHARMACIST OR OTHER HEALTH CARE PROVIDER.
For more information, call 1-888-235-BION or 1-888-235-2466.
This Patient information has been approved by the U.S. Food and Drug Administration.
Distributed by:
Bionpharma Inc.
Princeton, NJ 08540
MADE IN INDIA
Revised: 05/24
FDA-03
948026844
-
PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
NDC: 69452-330-20
Terazosin Capsules, USP
1 mg
Rx only
100 Capsules

NDC: 69452-331-20
Terazosin Capsules, USP
2 mg
Rx only
100 Capsules

NDC: 69452-332-20
Terazosin Capsules, USP
5 mg
Rx only
100 Capsules

NDC: 69452-333-20
Terazosin Capsules, USP
10 mg
Rx only
100 Capsules

- 1 mg-1000's count
- 2 mg- 1000's count
- 5 mg- 1000's count
- 10 mg- 1000's count
-
INGREDIENTS AND APPEARANCE
TERAZOSIN
terazosin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69452-330 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERAZOSIN HYDROCHLORIDE (UNII: D32S14F082) (TERAZOSIN - UNII:8L5014XET7) TERAZOSIN 1 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) SODIUM LAURYL SULFATE (UNII: 368GB5141J) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color gray (gray opaque cap and gray opaque body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code TE1 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-330-20 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/11/2024 2 NDC: 69452-330-32 1000 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075667 03/11/2024 TERAZOSIN
terazosin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69452-331 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERAZOSIN HYDROCHLORIDE (UNII: D32S14F082) (TERAZOSIN - UNII:8L5014XET7) TERAZOSIN 2 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) GELATIN (UNII: 2G86QN327L) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color white (ivory opaque cap and ivory opaque body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code TE2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-331-20 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/11/2024 2 NDC: 69452-331-32 1000 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075667 03/11/2024 TERAZOSIN
terazosin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69452-332 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERAZOSIN HYDROCHLORIDE (UNII: D32S14F082) (TERAZOSIN - UNII:8L5014XET7) TERAZOSIN 5 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C RED NO. 40 (UNII: WZB9127XOA) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) D&C RED NO. 28 (UNII: 767IP0Y5NH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) AMMONIA (UNII: 5138Q19F1X) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) Product Characteristics Color orange (orange opaque cap and orange opaque body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code TE3 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-332-20 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/11/2024 2 NDC: 69452-332-32 1000 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075667 03/11/2024 TERAZOSIN
terazosin capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 69452-333 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TERAZOSIN HYDROCHLORIDE (UNII: D32S14F082) (TERAZOSIN - UNII:8L5014XET7) TERAZOSIN 10 mg Inactive Ingredients Ingredient Name Strength LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STARCH, CORN (UNII: O8232NY3SJ) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MAGNESIUM STEARATE (UNII: 70097M6I30) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) GELATIN (UNII: 2G86QN327L) SHELLAC (UNII: 46N107B71O) FERROSOFERRIC OXIDE (UNII: XM0M87F357) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) AMMONIA (UNII: 5138Q19F1X) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) Product Characteristics Color green (green opaque cap and green opaque body) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code TE4 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69452-333-20 100 in 1 BOTTLE; Type 0: Not a Combination Product 03/11/2024 2 NDC: 69452-333-32 1000 in 1 BOTTLE; Type 0: Not a Combination Product 12/20/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA075667 03/11/2024 Labeler - Bionpharma Inc. (079637826) Registrant - Bionpharma Inc. (079637826) Establishment Name Address ID/FEI Business Operations OrBion Pharmaceuticals Private Limited 854403569 manufacture(69452-331, 69452-330, 69452-332, 69452-333)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.