Acetaminophen Rapid Release Gelcaps
Acetaminophen by
Drug Labeling and Warnings
Acetaminophen by is a Other medication manufactured, distributed, or labeled by Granules India Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
ACETAMINOPHEN RAPID RELEASE- acetaminophen capsule, gelatin coated
Granules India Limited
----------
Acetaminophen Rapid Release Gelcaps
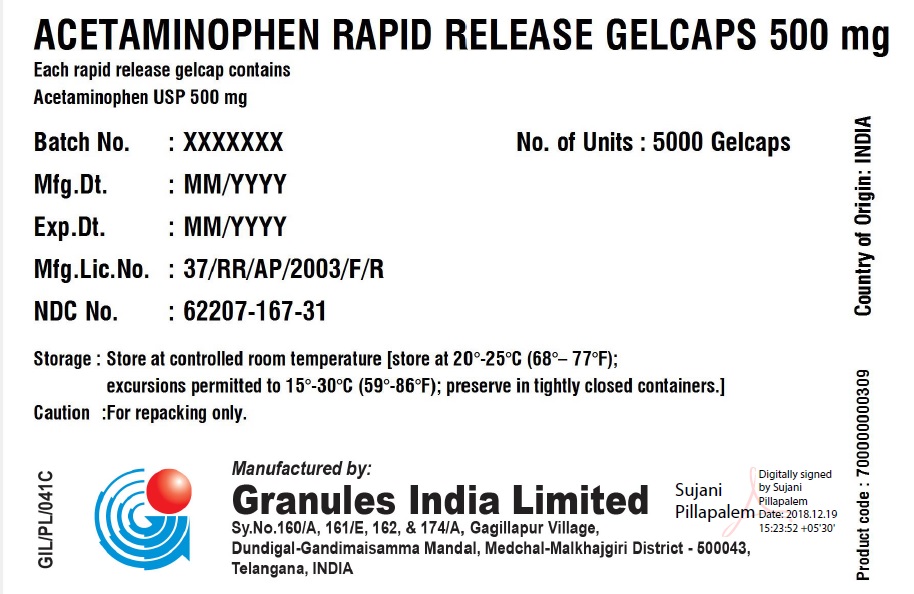
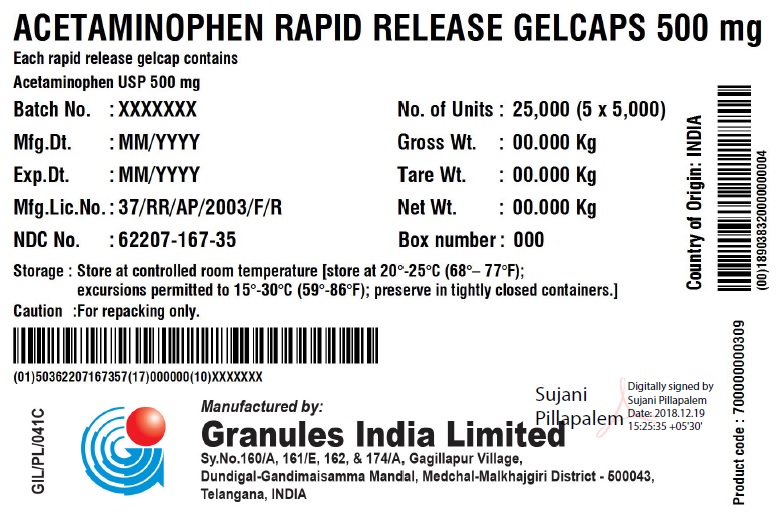
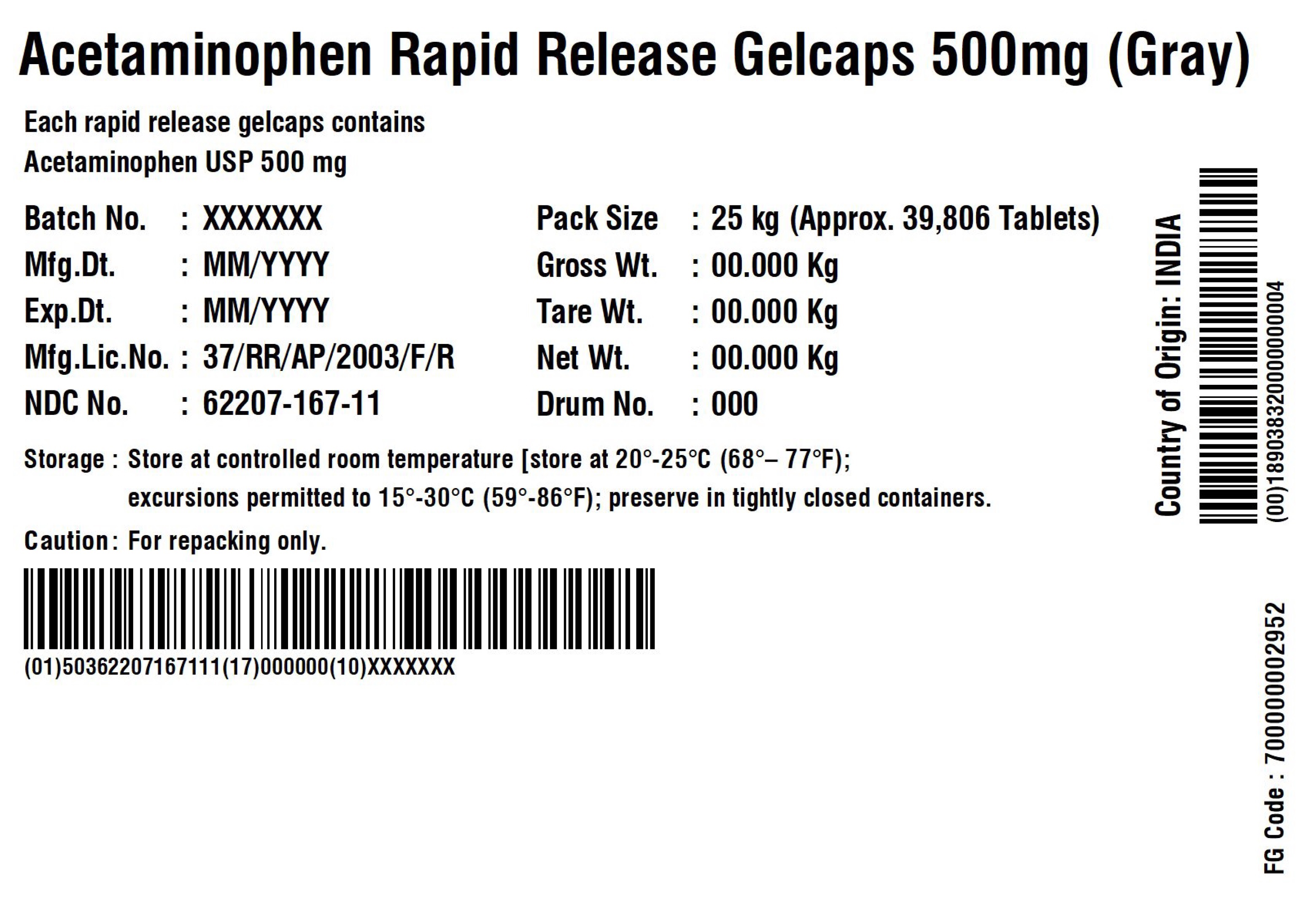
ACETAMINOPHEN RAPID RELEASE GELCAPS 500 MG
Each rapid release gelcap contains
Acetaminophen USP 500 mg
| Batch No.: | XXX | No. of Units | 5 x 5000 Gelcaps |
| Mfg. Dt.: | MMM/YYYY | Gross Wt.: | 0.00 kg |
| Exp. Dt.: | MMM/YYYY | Tare Wt.: | 0.00 kg |
| Mfg. Lic. No.: | 37/RR/AP/2003/F/R | Net Wt.: | 0.00 kg |
| NDC No. | XXXXX-XXX-XX | Box number: | 000 |
| Storage: | Store at 20 – 25C (68 – 77F); excursions permitted to 15 – 30 C (59 – 86F); Preserve in tightly closed containers. | ||
| Caution | For Repacking only. | ||
Manufactured in India by:
Granules India Limited
Plot No. 160/A, 161/E, Gagillapur Village
Qutbullapur Mandal, Ranga Reddy Dt – 500 043, AP.
| ACETAMINOPHEN
RAPID RELEASE
acetaminophen capsule, gelatin coated |
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - Granules India Limited (915000087) |
| Registrant - Granules India Limited (915000087) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Granules India Limited | 918609236 | manufacture(62207-167) , analysis(62207-167) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GRANULES LIFE SCIENCES PRIVATE LIMITED | 644776835 | manufacture(62207-167) , pack(62207-167) , label(62207-167) | |
Revised: 8/2024
Document Id: 1fa1ee2f-e3eb-f32e-e063-6394a90ae940
Set id: 140244ec-5bd2-4713-a491-457c4dd90fcb
Version: 10
Effective Time: 20240814
Trademark Results [Acetaminophen]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ACETAMINOPHEN 85615223 not registered Dead/Abandoned |
General Merchandise importers and Expoters 2012-05-03 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.