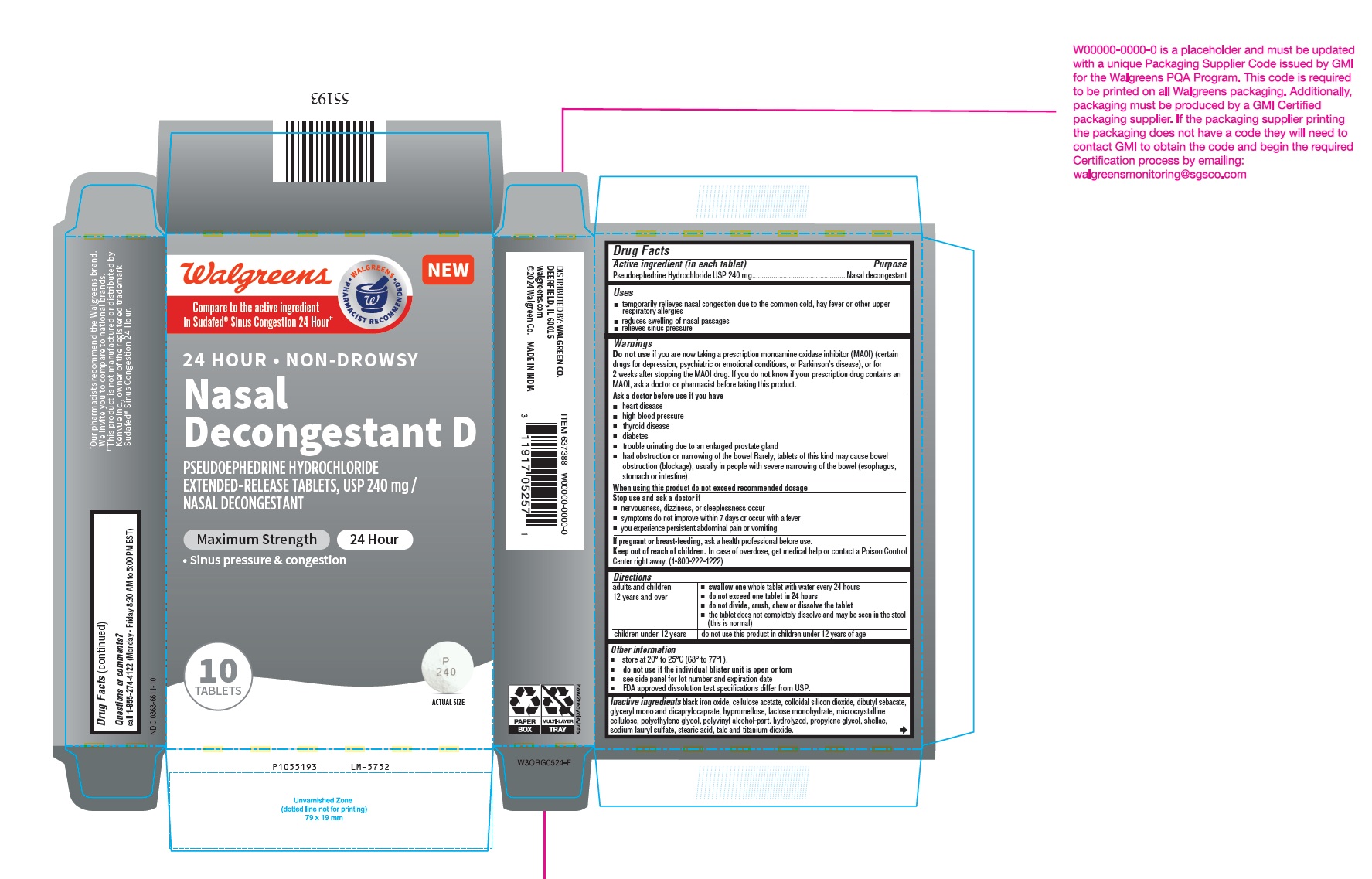
PSEUDOEPHEDRINE HYDROCHLORIDE tablet, extended release
Pseudoephedrine Hydrochloride by
Drug Labeling and Warnings
Pseudoephedrine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by WALGREEN CO., Aurohealth LLC, Aurobindo Pharma Limited. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Purpose
- Uses
-
Warnings
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
-
Ask a doctor before use if you have
- heart disease
- high blood pressure
- thyroid disease
- diabetes
- trouble urinating due to an enlarged prostate gland
- had obstruction or narrowing of the bowel Rarely, tablets of this kind may cause bowel obstruction (blockage), usually in people with severe narrowing of the bowel (esophagus, stomach or intestine).
- When using this product do not exceed recommended dosage
- STOP USE
- PREGNANCY OR BREAST FEEDING
- Keep out of reach of children.
-
Directions
adults and children
12 years and over
- swallow one whole tablet with water every 24 hours
- do not exceed one tablet in 24 hours
- do not divide, crush, chew or dissolve the tablet
- the tablet does not completely dissolve and may be seen in the stool (this is normal)
children under 12 years
do not use this product in children under 12 years of age
- Other information
-
Inactive ingredients
black iron oxide, cellulose acetate, colloidal silicon dioxide, dibutyl sebacate, glyceryl mono and dicaprylocaprate, hypromellose, lactose monohydrate, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol-part. hydrolyzed, propylene glycol, shellac, sodium lauryl sulfate, stearic acid, talc and titanium dioxide.
Questions or comments?
call 1-855-274-4122 (Monday - Friday 8:30 AM to 5:00 PM EST)
DISTRIBUTED BY: WALGREEN CO.
DEERFIELD, IL 60015
MADE IN INDIA
- PACKAGE LABEL-PRINCIPAL DISPLAY PANEL - 1 x 10 Blister Carton Label
-
INGREDIENTS AND APPEARANCE
PSEUDOEPHEDRINE HYDROCHLORIDE
pseudoephedrine hydrochloride tablet, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0363-6611 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PSEUDOEPHEDRINE HYDROCHLORIDE (UNII: 6V9V2RYJ8N) (PSEUDOEPHEDRINE - UNII:7CUC9DDI9F) PSEUDOEPHEDRINE HYDROCHLORIDE 240 mg Inactive Ingredients Ingredient Name Strength FERROSOFERRIC OXIDE (UNII: XM0M87F357) CELLULOSE ACETATE (UNII: 3J2P07GVB6) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) DIBUTYL SEBACATE (UNII: 4W5IH7FLNY) GLYCERYL MONO- AND DICAPRYLOCAPRATE (UNII: U72Q2I8C85) HYPROMELLOSE 2208 (100000 MPA.S) (UNII: VM7F0B23ZI) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SHELLAC (UNII: 46N107B71O) SODIUM LAURYL SULFATE (UNII: 368GB5141J) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color WHITE (white to off-white) Score no score Shape ROUND (biconvex) Size 11mm Flavor Imprint Code P240 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0363-6611-10 1 in 1 CARTON 01/30/2026 1 10 in 1 BLISTER PACK; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA218854 01/30/2026 Labeler - WALGREEN CO. (008965063) Registrant - Aurohealth LLC (078728447) Establishment Name Address ID/FEI Business Operations Aurobindo Pharma Limited 650381903 ANALYSIS(0363-6611) , MANUFACTURE(0363-6611)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.