ALLERGY- diphenhydramine hydrochloride liquid
Allergy by
Drug Labeling and Warnings
Allergy by is a Otc medication manufactured, distributed, or labeled by Accudial Pharmaceutical, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 5 mL)1
- Purpose
- Uses
-
Warnings
Do not use
- to make a child sleepy
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if the child has
- a breathing problem such as chronic bronchitis
- glaucoma
- a sodium-restricted diet
-
Directions
- take every 4 to 6 hours
- do not take more than 6 doses in 24 hours
- to find right dose, use rotating bottle label to dose by weight; otherwise, use chart below to dose by age
- specifically designed for use with enclosed dosing spoon. Use only enclosed dosing spoon to dose this product. Do not use any other dosing device
children under 2 years do not use children 2 to 5 years do not use unless directed by a doctor children 6 to under 12 years 5 mL to 10 mL
(1 to 2 teaspoonfuls) - Other information
- Inactive ingredients
- Questions?
-
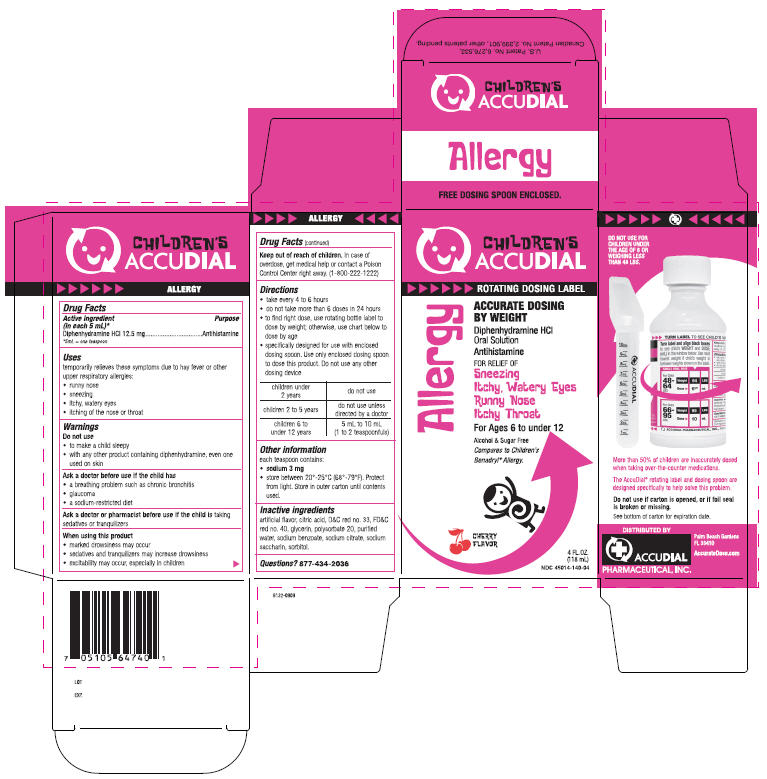
PRINCIPAL DISPLAY PANEL - 118 mL Carton
CHILDREN'S
ACCUDIALROTATING DOSING LABEL
ACCURATE DOSING
BY WEIGHTDiphenhydramine HCl
Oral Solution
AnthistamineFOR RELIEF OF
Sneezing
Itchy, Watery Eyes
Runny Nose
Itchy ThroatFor Ages 6 to under 12
Alcohol & Sugar Free
Compares to Children's
Benadryl® Allergy.CHERRY
FLAVOR4 FL. OZ.
(118 mL)
NDC: 45014-140-04Allergy
- SPL UNCLASSIFIED SECTION
-
INGREDIENTS AND APPEARANCE
ALLERGY
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 45014-140 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Diphenhydramine Hydrochloride (UNII: TC2D6JAD40) (Diphenhydramine - UNII:8GTS82S83M) Diphenhydramine Hydrochloride 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength Citric Acid Monohydrate (UNII: 2968PHW8QP) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) Glycerin (UNII: PDC6A3C0OX) Polysorbate 20 (UNII: 7T1F30V5YH) Water (UNII: 059QF0KO0R) Sodium Benzoate (UNII: OJ245FE5EU) Sodium Citrate (UNII: 1Q73Q2JULR) Saccharin Sodium (UNII: SB8ZUX40TY) Sorbitol (UNII: 506T60A25R) Product Characteristics Color PINK Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 45014-140-04 118 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 10/26/2009 Labeler - Accudial Pharmaceutical, Inc. (831999201)
Trademark Results [Allergy]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ALLERGY 97069656 not registered Live/Pending |
Rodriguez, Kent J 2021-10-12 |
 ALLERGY 90819383 not registered Live/Pending |
Rodriguez, Kent J 2021-07-09 |
 ALLERGY 87534443 5406627 Live/Registered |
RSM Medical Inc. 2017-07-19 |
 ALLERGY 87518600 5400551 Live/Registered |
RSM Medical Inc. 2017-07-06 |
 ALLERGY 74392251 not registered Dead/Abandoned |
Danta, Inc. 1993-05-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.