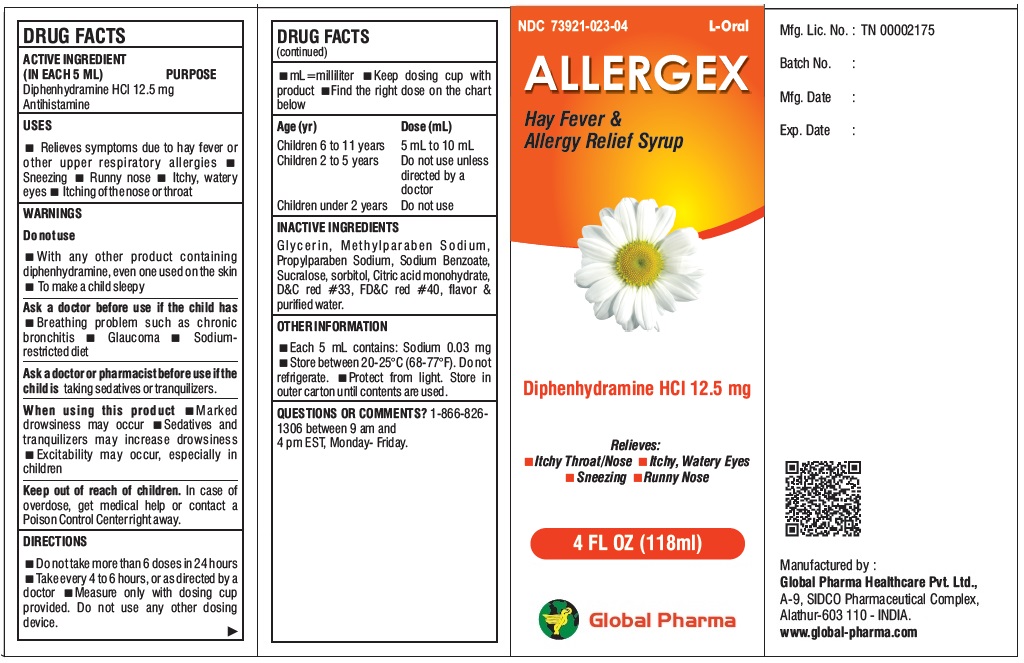
L-Oral ALLERGEX Hay Fever & Allergy Relief Syrup
L-Oral ALLERGEX Hay Fever and Allergy Relief Syrup by
Drug Labeling and Warnings
L-Oral ALLERGEX Hay Fever and Allergy Relief Syrup by is a Otc medication manufactured, distributed, or labeled by GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
L-ORAL ALLERGEX HAY FEVER AND ALLERGY RELIEF SYRUP- diphenhydramine hydrochloride syrup
GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
L-Oral ALLERGEX Hay Fever & Allergy Relief Syrup
USES
Relieves symptoms due to hay fever or other upper respiratory allergies Sneezing Runny nose Itchy, watery eyes Itching of the nose or throat
WARNINGS
Do not use
With any other product containing diphenhydramine, even one used on the skin To make a child sleepy
Ask a doctor before use if the child has Breathing problem such as chronic bronchitis Glaucoma Sodium-restricted diet
Ask a doctor or pharmacist before use if the child is taking sedatives or tranquilizers.
When using this product Marked drowsiness may occur Sedatives and tranquilizers may increase drowsiness Excitability may occur, especially in children
DIRECTIONS
Do not take more than 6 doses in 24 hours Take every 4 to 6 hours, or as directed by a doctor Measure only with dosing cup provided. Do not use any other dosing device. mL=milliliter Keep dosing cup with product Find the right dose on the chart below
Age (yr) Dose (mL)
Children 6 to 11 years 5 mL to 10 mL
Children 2 to 5 years Do not use unless directed by a doctor
Children under 2 years Do not use
INACTIVE INGREDIENTS
Glycerin, Methylparaben Sodium, Propylparaben Sodium, Sodium Benzoate, Sucralose, sorbitol, Citric acid monohydrate, D&C red #33, FD&C red #40, flavor & purified water.
OTHER INFORMATION
Each 5 mL contains: Sodium 0.03 mg Store between 20-25°C (68-77°F). Do not refrigerate. Protect from light. Store in outer carton until contents are used.
| L-ORAL ALLERGEX HAY FEVER AND ALLERGY RELIEF SYRUP
diphenhydramine hydrochloride syrup |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED (860186917) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| GLOBAL PHARMA HEALTHCARE PRIVATE LIMITED | 860186917 | manufacture(73921-023) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.