KAOPECTATE- bismuth subsalicylate liquid
Kaopectate by
Drug Labeling and Warnings
Kaopectate by is a Otc medication manufactured, distributed, or labeled by KRAMER LABORATORIES. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active Ingredient (per 15 mL)
- Purposes
- Uses
-
Warnings
Reye's syndrome: Children and teenagers who have or are recovering from chicken pox or flu-like symptoms should not use this product. When using this product, if changes in behavior with nausea and vomiting occur, consult a doctor because these symptoms could be an early sign of Reye's syndrome, a rare but serious illness.
Allergy Alert: Contains salicylate. - Do not take if you are
- Do not use if you have
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- When using this product
- Stop use and ask a doctor if
- If pregnant or breast-feeding
- Keep out of reach of children
-
Directions
- shake well immediately before each use
- only use pre-measured dose cup
- adults and children 12 years of age and older: 1 dose (30 mL) every hour as needed.
- do not exceed 4 doses (120 mL) in 24 hours
- use until diarrhea stops but not more than 2 days
- children under 12 years: ask a doctor
- drink plenty of clear fluids to help prevent dehydration caused by diarrhea
- Other information
- Inactive ingredients
-
Principal Display Panel
EXTRA
STRENGTH
2X
THE MEDICINE
PER DOSE
Kaopectate®
Bismuth Subsalicylate 525 mg
● Anti-Diarrheal ● Upset stomach Reliever
Diarrhea &
Upset Stomach
√ Begins controlling symptoms from the first dose
√ Twice the medicine as regular strength Kaopectate
√ Quickly relieves urgency, gas, and cramping
11 fl oz (325 mL)
Peppermint Flavor
682PPT11KA0LB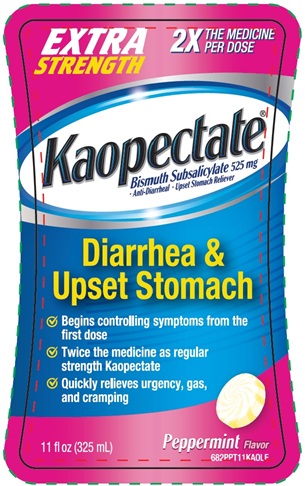
DO NOT USE IF INNER SEAL IS BROKEN OR MISSING
Kramer Laboratories, Bridgewater, NJ 08807 1-800-824-4894
K0825 682PPT11KA0LB
Lot:
Exp:
PEEL CORNER TO READ
DRUG FACTS AND INFORMATION


-
INGREDIENTS AND APPEARANCE
KAOPECTATE
bismuth subsalicylate liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 55505-229 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Bismuth Subsalicylate (UNII: 62TEY51RR1) (Salicylic Acid - UNII:O414PZ4LPZ, Bismuth Cation - UNII:ZS9CD1I8YE) Bismuth Subsalicylate 525 mg in 15 mL Inactive Ingredients Ingredient Name Strength Caramel (UNII: T9D99G2B1R) Carboxymethylcellulose Sodium, Unspecified (UNII: K679OBS311) Fd&C Red No. 40 (UNII: WZB9127XOA) Microcrystalline Cellulose (UNII: OP1R32D61U) Sodium Salicylate (UNII: WIQ1H85SYP) Sorbic Acid (UNII: X045WJ989B) Sucrose (UNII: C151H8M554) Water (UNII: 059QF0KO0R) Xanthan Gum (UNII: TTV12P4NEE) Product Characteristics Color Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 55505-229-64 325 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/01/2026 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M008 02/01/2026 Labeler - KRAMER LABORATORIES (122720675)
Trademark Results [Kaopectate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 KAOPECTATE 71379981 0340742 Live/Registered |
UPJOHN COMPANY, THE 1936-06-19 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.