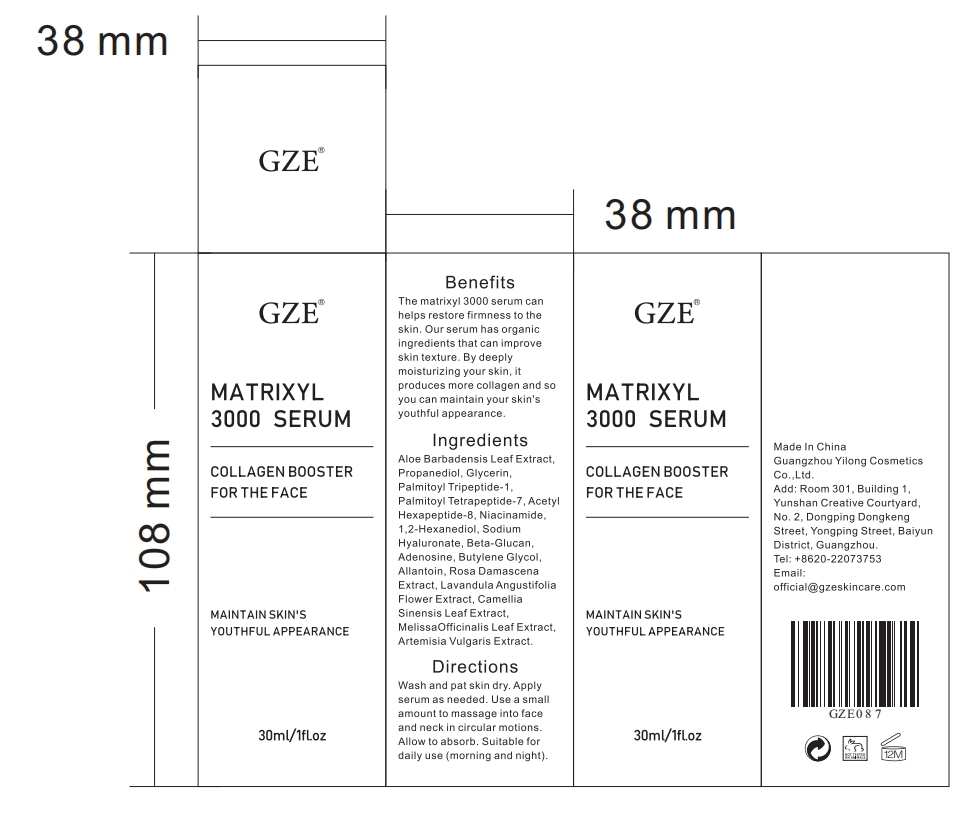
Gze MATRIXYL 3000 SERUM by Guangzhou Yilong Cosmetics Co.,Ltd. Gze MATRIXYL 3000 SERUM
Gze MATRIXYL 3000 SERUM by
Drug Labeling and Warnings
Gze MATRIXYL 3000 SERUM by is a Otc medication manufactured, distributed, or labeled by Guangzhou Yilong Cosmetics Co.,Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GZE MATRIXYL 3000 SERUM- palmitoyl tripeptide-1 liquid
Guangzhou Yilong Cosmetics Co.,Ltd.
----------
Gze MATRIXYL 3000 SERUM
ALLANTOIN
NIACINAMIDE
ADENOSINE
GLYCERIN
PEG-9 DIGLYCIDYL ETHERISODIUM HYALURONATE CROSSPOLYMER
1.2-HEXANEDIOL
PROPANEDIOL
1.3-BETA-GLUCAN SYNTHASE COMPONENT FKS1
BUTYLENE GLYCOL
ROSA DAMASCENA FLOWER
ALOE ANDONGENSIS LEAF
LAVENDER OIL
CAMELLIA SINENSIS WHOLE
Wash and pat skin dry. Apply serum as needed.Use a small amount to massage into face and neck in circular motions. Allow to absorb. Suitable for daily use (morning and night).
| GZE MATRIXYL 3000 SERUM
palmitoyl tripeptide-1 liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Guangzhou Yilong Cosmetics Co.,Ltd. (712647107) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Guangzhou Yilong Cosmetics Co.,Ltd. | 712647107 | manufacture(83566-087) | |
Revised: 7/2025
Document Id: 39f27fa3-e6c0-0cfd-e063-6394a90a1c38
Set id: 18f4abd0-b0c8-6fd1-e063-6294a90a1807
Version: 3
Effective Time: 20250715