SILENOR- doxepin hydrochloride tablet
Silenor by
Drug Labeling and Warnings
Silenor by is a Prescription medication manufactured, distributed, or labeled by Currax Pharmaceuticals LLC, Patheon Pharmaceuticals, Inc, Plantex Ltd, Teva API India Private Ltd., Ropack Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use Silenor safely and effectively. See full prescribing information for Silenor.
Silenor® (doxepin) tablets for oral administration
Initial U.S. Approval: 1969INDICATIONS AND USAGE
DOSAGE AND ADMINISTRATION
DOSAGE FORMS AND STRENGTHS
- 3 mg and 6 mg tablets. Tablets not scored. (3)
CONTRAINDICATIONS
- Hypersensitivity to doxepin hydrochloride, inactive ingredients, or other dibenzoxepines. (4.1)
- Co-administration with Monoamine Oxidase Inhibitors (MAOIs): Do not administer if patient is taking MAOIs or has used MAOIs within the past two weeks. (4.2)
- Untreated narrow angle glaucoma or severe urinary retention. (4.3)
WARNINGS AND PRECAUTIONS
- Need to Evaluate for Co-morbid Diagnoses: Reevaluate if insomnia persists after 7 to 10 days of use. (5.1)
- Abnormal thinking, behavioral changes, complex behaviors: May include "Sleep-driving" and hallucinations. Immediately evaluate any new onset behavioral changes. (5.2)
- Depression: Worsening of depression or suicidal thinking may occur. Prescribe the least amount feasible to avoid intentional overdose. (5.3)
- CNS-depressant effects: Use can impair alertness and motor coordination. Avoid engaging in hazardous activities such as operating a motor vehicle or heavy machinery after taking drug. (5.4) Do not use with alcohol. (5.4, 7.3)
- Potential additive effects when used in combination with CNS depressants or sedating antihistamines. Dose reduction may be needed. (5.4, 7.4)
- Patients with severe sleep apnea: Silenor is ordinarily not recommended for use in this population. (8.7)
ADVERSE REACTIONS
- The most common treatment-emergent adverse reactions, reported in ≥ 2% of patients treated with Silenor, and more commonly than in patients treated with placebo, were somnolence/sedation, nausea, and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Currax Pharmaceuticals LLC at 1-800-793-2145 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- MAO inhibitors: Silenor should not be administered in patients on MAOIs within the past two weeks. (4.2)
- Cimetidine: Increases exposure to doxepin. (7.2)
- Alcohol: Sedative effects may be increased with doxepin. (7.3, 5.4)
- CNS Depressants and Sedating Antihistamines: Sedative effects may be increased with doxepin. (7.4, 5.4)
- Tolazamide: A case of severe hypoglycemia has been reported. (7.5)
USE IN SPECIFIC POPULATIONS
- Pregnancy: Based on animal data, may cause fetal harm. (8.1)
- Nursing Mothers: Infant exposure via human milk. (8.3)
- Pediatric Use: Safety and effectiveness have not been evaluated. (8.4)
- Geriatric Use: The recommended starting dose is 3 mg. Monitor prior to considering dose escalation. (2.2, 8.5)
- Use in Patients with Comorbid Illness: Initiate treatment with 3 mg in patients with hepatic impairment or tendency to urinary retention. (8.6, 4.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1. INDICATIONS AND USAGE
2. DOSAGE AND ADMINISTRATION
2.1. Dosing in Adults
2.2. Dosing in the Elderly
2.3. Administration
3. DOSAGE FORMS AND STRENGTHS
4. CONTRAINDICATIONS
4.1. Hypersensitivity
4.2. Co-administration with Monoamine Oxidase Inhibitors (MAOIs)
4.3. Glaucoma and Urinary Retention
5. WARNINGS AND PRECAUTIONS
5.1. Need to Evaluate for Comorbid Diagnoses
5.2. Abnormal Thinking and Behavioral Changes
5.3. Suicide Risk and Worsening of Depression
5.4. CNS Depressant Effects
6. ADVERSE REACTIONS
6.1. Clinical Trials Experience
6.2. Studies Pertinent to Safety Concerns for Sleep-promoting Drugs
6.3. Other Reactions Observed During the Pre-marketing Evaluation of Silenor
7. DRUG INTERACTIONS
7.1. Cytochrome P450 Isozymes
7.2. Cimetidine
7.3. Alcohol
7.4. CNS Depressants and Sedating Antihistamines
7.5. Tolazamide
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
8.2. Labor and Delivery
8.3. Nursing Mothers
8.4. Pediatric Use
8.5. Geriatric Use
8.6. Use in Patients with Hepatic Impairment
8.7. Use in Patients with Sleep Apnea
9. DRUG ABUSE AND DEPENDENCE
9.1. Controlled Substance
9.2. Abuse
9.3. Dependence
10. OVERDOSAGE
10.1. Signs and Symptoms of Excessive Doses
10.2. Signs and Symptoms of Critical Overdose
10.3. Recommended Management
11. DESCRIPTION
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
12.2. Pharmacodynamics
12.3. Pharmacokinetics
12.4. Drug Interactions
12.5. Special Populations
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
14. CLINICAL STUDIES
14.1. Controlled Clinical Trials
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
16.2. Storage and Handling
17. PATIENT COUNSELING INFORMATION
17.1. Sleep-driving and Other Complex Behaviors
17.2. Suicide risk and Worsening of Depression:
17.3. Administration Instructions
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1. INDICATIONS AND USAGE
Silenor is indicated for the treatment of insomnia characterized by difficulty with sleep maintenance. The clinical trials performed in support of efficacy were up to 3 months in duration [see Clinical Studies (14)].
-
2. DOSAGE AND ADMINISTRATION
The dose of Silenor should be individualized.
2.1. Dosing in Adults
The recommended dose of Silenor for adults is 6 mg once daily. A 3 mg once daily dose may be appropriate for some patients, if clinically indicated.
2.2. Dosing in the Elderly
The recommended starting dose of Silenor in elderly patients (≥ 65 years old) is 3 mg once daily. The daily dose can be increased to 6 mg, if clinically indicated.
2.3. Administration
Silenor should be taken within 30 minutes of bedtime.
To minimize the potential for next day effects, Silenor should not be taken within 3 hours of a meal [see Clinical Pharmacology (12.3)].
The total Silenor dose should not exceed 6 mg per day.
- 3. DOSAGE FORMS AND STRENGTHS
-
4. CONTRAINDICATIONS
4.1. Hypersensitivity
Silenor is contraindicated in individuals who have shown hypersensitivity to doxepin HCl, any of its inactive ingredients, or other dibenzoxepines.
4.2. Co-administration with Monoamine Oxidase Inhibitors (MAOIs)
Serious side effects and even death have been reported following the concomitant use of certain drugs with MAO inhibitors. Do not administer Silenor if patient is currently on MAOIs or has used MAOIs within the past two weeks. The exact length of time may vary depending on the particular MAOI dosage and duration of treatment.
-
5. WARNINGS AND PRECAUTIONS
5.1. Need to Evaluate for Comorbid Diagnoses
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Exacerbation of insomnia or the emergence of new cognitive or behavioral abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with hypnotic drugs.
5.2. Abnormal Thinking and Behavioral Changes
Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a hypnotic, with amnesia for the event) have been reported with hypnotics. These events can occur in hypnotic-naive as well as in hypnotic-experienced persons. Although behaviors such as "sleep-driving" may occur with hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with hypnotics appears to increase the risk of such behaviors, as does the use of hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of Silenor should be strongly considered for patients who report a "sleep-driving" episode. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a hypnotic. As with "sleep-driving", patients usually do not remember these events. Amnesia, anxiety and other neuro-psychiatric symptoms may occur unpredictably.
5.3. Suicide Risk and Worsening of Depression
In primarily depressed patients, worsening of depression, including suicidal thoughts and actions (including completed suicides), has been reported in association with the use of hypnotics.
Doxepin, the active ingredient in Silenor, is an antidepressant at doses 10- to 100-fold higher than in Silenor. Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies of major depressive disorder (MDD) and other psychiatric disorders. Risk from the lower dose of doxepin in Silenor can not be excluded.
It can rarely be determined with certainty whether a particular instance of the abnormal behaviors listed above is drug induced, spontaneous in origin, or a result of an underlying psychiatric or physical disorder. Nonetheless, the emergence of any new behavioral sign or symptom of concern requires careful and immediate evaluation.
5.4. CNS Depressant Effects
After taking Silenor, patients should confine their activities to those necessary to prepare for bed. Patients should avoid engaging in hazardous activities, such as operating a motor vehicle or heavy machinery, at night after taking Silenor, and should be cautioned about potential impairment in the performance of such activities that may occur the day following ingestion.
When taken with Silenor, the sedative effects of alcoholic beverages, sedating antihistamines, and other CNS depressants may be potentiated [see Warnings and Precautions (5.2) and Drug Interactions (7.3, 7.4)]. Patients should not consume alcohol with Silenor [see Warnings and Precautions (5.2) and Drug Interactions (7.3)]. Patients should be cautioned about potential additive effects of Silenor used in combination with CNS depressants or sedating antihistamines [see Warnings and Precautions (5.2) and Drug Interactions (7.4)].
-
6. ADVERSE REACTIONS
The following serious adverse reactions are discussed in greater detail in other sections of labeling:
- Abnormal thinking and behavioral changes [see Warnings and Precautions (5.2)].
- Suicide risk and worsening of depression [see Warnings and Precautions (5.3)].
- CNS Depressant effects [see Warnings and Precautions (5.4)].
6.1. Clinical Trials Experience
The pre-marketing development program for Silenor included doxepin HCl exposures in 1017 subjects (580 insomnia patients and 437 healthy subjects) from 12 studies conducted in the United States. 863 of these subjects (580 insomnia patients and 283 healthy subjects) participated in six randomized, placebo-controlled efficacy studies with Silenor doses of 1 mg, 3 mg, and 6 mg for up to 3-months in duration.
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice. However, data from the Silenor studies provide the physician with a basis for estimating the relative contributions of drug and non-drug factors to adverse reaction incidence rates in the populations studied.
Associated with Discontinuation of Treatment
The percentage of subjects discontinuing Phase 1, 2, and 3 trials for an adverse reaction was 0.6% in the placebo group compared to 0.4%, 1.0%, and 0.7% in the Silenor 1 mg, 3 mg, and 6 mg groups, respectively. No reaction that resulted in discontinuation occurred at a rate greater than 0.5%.
Adverse Reactions Observed at an Incidence of ≥ 2% in Controlled Trials
Table 1 shows the incidence of treatment-emergent adverse reactions from three long-term (28 to 85 days) placebo-controlled studies of Silenor in adult (N=221) and elderly (N=494) subjects with chronic insomnia.
Reactions reported by Investigators were classified using a modified MedDRA dictionary of preferred terms for purposes of establishing incidence. The table includes only reactions that occurred in 2% or more of subjects who received Silenor 3 mg or 6 mg in which the incidence in subjects treated with Silenor was greater than the incidence in placebo-treated subjects.
Table 1 Incidence (%) of Treatment-Emergent Adverse Reactions in Long-term Placebo-Controlled Clinical Trials System Organ Class
Preferred Term*Placebo
(N=278)Silenor
3 mg
(N=157)Silenor
6 mg
(N=203)- * Includes reactions that occurred at a rate of ≥ 2% in any Silenor-treated group and at a higher rate than placebo.
Nervous System Disorders Somnolence/Sedation 4 6 9 Infections and Infestations Upper Respiratory Tract Infection/Nasopharyngitis 2 4 2 Gastroenteritis 0 2 0 Gastrointestinal Disorders Nausea 1 2 2 Vascular Disorders Hypertension 0 3 < 1 The most common treatment-emergent adverse reaction in the placebo and each of the Silenor dose groups was somnolence/sedation.
6.2. Studies Pertinent to Safety Concerns for Sleep-promoting Drugs
Residual Pharmacological Effect in Insomnia Trials
Five randomized, placebo-controlled studies in adults and the elderly assessed next-day psychomotor function within 1 hour of awakening utilizing the digit-symbol substitution test (DSST), symbol copying test (SCT), and visual analog scale (VAS) for sleepiness, following night time administration of Silenor.
In a one-night, double-blind study conducted in 565 healthy adult subjects experiencing transient insomnia, Silenor 6 mg showed modest negative changes in SCT and VAS.
In a 35-day, double-blind, placebo-controlled, parallel group study of Silenor 3 and 6 mg in 221 adults with chronic insomnia, small decreases in the DSST and SCT occurred in the 6 mg group.
In a 3-month, double-blind, placebo-controlled, parallel group study in 240 elderly subjects with chronic insomnia, Silenor 1 mg and 3 mg was comparable to placebo on DSST, SCT, and VAS.
6.3. Other Reactions Observed During the Pre-marketing Evaluation of Silenor
Silenor was administered to 1017 subjects in clinical trials in the United States. Treatment-emergent adverse reactions recorded by clinical investigators were standardized using a modified MedDRA dictionary of preferred terms. The following is a list of MedDRA terms that reflect treatment-emergent adverse reactions reported by subjects treated with Silenor.
Adverse reactions are further categorized by body system and listed in order of decreasing frequency according to the following definitions: Frequent adverse reactions are those that occurred on one or more occasions in at least 1/100 subjects; Infrequent adverse reactions are those that occurred in fewer than 1/100 subjects and more than 1/1000 subjects. Rare adverse reactions are those that occurred in fewer than 1/1000 subjects. Adverse reactions that are listed in Table 1 are not included in the following listing of frequent, infrequent, and rare AEs.
Blood and Lymphatic System Disorders: Infrequent: anemia; Rare: thrombocythemia.
Cardiac Disorders: Rare: atrioventricular block, palpitations, tachycardia, ventricular extrasystoles.
Ear and Labyrinth Disorders: Rare: ear pain, hypoacusis, motion sickness, tinnitus, tympanic membrane perforation.
Eye Disorders: Infrequent: eye redness, vision blurred; Rare: blepharospasm, diplopia, eye pain, lacrimation decreased.
Gastrointestinal Disorders: Infrequent: abdominal pain, dry mouth, gastroesophageal reflux disease, vomiting; Rare: dyspepsia, constipation, gingival recession, haematochezia, lip blister.
General Disorders and Administration Site Conditions: Infrequent: asthenia, chest pain, fatigue; Rare: chills, gait abnormal, edema peripheral.
Hepatobiliary Disorders: Rare: hyperbilirubinemia.
Immune System Disorders: Rare: hypersensitivity.
Infections and Infestations: Infrequent: bronchitis, fungal infection, laryngitis, sinusitis, tooth infection, urinary tract infection, viral infection; Rare: cellulitis staphylococcal, eye infection, folliculitis, gastroenteritis viral, herpes zoster, infective tenosynovitis, influenza, lower respiratory tract infection, onychomycosis, pharyngitis, pneumonia.
Injury, Poisoning and Procedural Complications: Infrequent: back injury, fall, joint sprain; Rare: bone fracture, skin laceration.
Investigations: Infrequent: blood glucose increased; Rare: alanine aminotransferase increased, blood pressure decreased, blood pressure increased, electrocardiogram ST-T segment abnormal, electrocardiogram QRS complex abnormal, heart rate decreased, neutrophil count decreased, QRS axis abnormal, transaminases increased.
Metabolism and Nutrition Disorders: Infrequent: anorexia, decreased appetite, hyperkalemia, hypermagnesemia, increased appetite; Rare: hypokalemia.
Musculoskeletal and Connective Tissue Disorders: Infrequent: arthralgia, back pain, myalgia, neck pain, pain in extremity; Rare: joint range of motion decreased, muscle cramp, sensation of heaviness.
Neoplasms Benign, Malignant and Unspecified (Including Cysts and Polyps): Rare: lung adenocarcinoma stage I, malignant melanoma.
Nervous System Disorders: Frequent: dizziness; Infrequent: dysgeusia, lethargy, parasthesia, syncope; Rare: ageusia, ataxia, cerebrovascular accident, disturbance in attention, migraine, sleep paralysis, syncope vasovagal, tremor.
Psychiatric Disorders: Infrequent: abnormal dreams, adjustment disorder, anxiety, depression; Rare: confusional state, elevated mood, insomnia, libido decreased, nightmare.
Reproductive System and Breast Disorders: Rare: breast cyst, dysmenorrhea.
Renal and Urinary Disorders: Rare: dysuria, enuresis, hemoglobinuria, nocturia.
Respiratory, Thoracic and Mediastinal Disorders: Infrequent: nasal congestion, pharyngolaryngeal pain, sinus congestion, wheezing; Rare: cough, crackles lung, nasopharyngeal disorder, rhinorrhea, dyspnea.
Skin and Subcutaneous Tissue Disorders: Infrequent: skin irritation; Rare: cold sweat, dermatitis, erythema, hyperhidrosis, pruritis, rash, rosacea.
Surgical and Medical Procedures: Rare: arthrodesis.
Vascular Disorders: Infrequent: pallor; Rare: blood pressure inadequately controlled, hematoma, hot flush.
In addition, the reactions below have been reported for other tricyclics and may be idiosyncratic (not related to dose).
Allergic: photosensitization, skin rash.
Hematologic: agranulocytosis, eosinophilia, leukopenia, purpura, thrombocytopenia.
-
7. DRUG INTERACTIONS
7.1. Cytochrome P450 Isozymes
Silenor is primarily metabolized by hepatic cytochrome P450 isozymes CYP2C19 and CYP2D6, and to a lesser extent, by CYP1A2 and CYP2C9. Inhibitors of these isozymes may increase the exposure of doxepin. Silenor is not an inhibitor of any CYP isozymes at therapeutically relevant concentrations. The ability of Silenor to induce CYP isozymes is not known.
7.2. Cimetidine
Silenor exposure is doubled with concomitant administration of cimetidine, a nonspecific inhibitor of CYP isozymes. A maximum dose of 3 mg is recommended in adults and elderly when cimetidine is co-administered with Silenor [see Clinical Pharmacology (12.4)]
7.3. Alcohol
When taken with Silenor, the sedative effects of alcohol may be potentiated [see Warnings and Precautions (5.2, 5.4)].
7.4. CNS Depressants and Sedating Antihistamines
When taken with Silenor, the sedative effects of sedating antihistamines and CNS depressants may be potentiated [see Warnings and Precautions (5.2, 5.4)].
-
8. USE IN SPECIFIC POPULATIONS
8.1. Pregnancy
Pregnancy Category C
There are no adequate and well-controlled studies of Silenor in pregnant women. Silenor should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Administration of doxepin to pregnant animals resulted in adverse effects on offspring development at doses greater than the maximum recommended human dose (MRHD) of 6 mg/day.
When doxepin (30, 100 and 150 mg/kg/day) was administered orally to pregnant rats during the period of organogenesis, developmental toxicity (increased incidences of fetal structural abnormalities and decreased fetal body weights) was noted at ≥100 mg/kg/day. The plasma exposures (AUC) at the no-effect dose for embryo-fetal developmental toxicity in rats (30 mg/kg/day) are approximately 6 and 3 times the plasma AUCs for doxepin and nordoxepin (the primary metabolite in humans), respectively, at the MRHD. When administered orally to pregnant rabbits (10, 30 and 60 mg/kg/day) during the period of organogenesis, fetal body weights were reduced at the highest dose in the absence of maternal toxicity. The plasma exposures (AUC) at the no-effect dose for developmental effects (30 mg/kg/day) are approximately 6 and 18 times the plasma AUCs for doxepin and nordoxepin, respectively, at the MRHD. Oral administration of doxepin (10, 30 and 100 mg/kg/day) to rats throughout the pregnancy and lactation periods resulted in decreased pup survival and transient growth delay at the highest dose. The plasma exposures (AUC) at the no-effect dose for adverse effects on pre- and postnatal development in rats (30 mg/kg/day) are approximately 3 and 2 times the plasma AUCs for doxepin and nordoxepin, respectively, at the MRHD.
8.3. Nursing Mothers
Doxepin is excreted in human milk after oral administration. There has been a report of apnea and drowsiness occurring in a nursing infant whose mother was taking the higher dose of doxepin used to treat depression. Caution should be exercised when Silenor is administered to nursing women.
8.4. Pediatric Use
The safety and effectiveness of Silenor in pediatric patients have not been evaluated.
8.5. Geriatric Use
A total of 362 subjects who were ≥ 65 years and 86 subjects who were ≥ 75 years received Silenor in controlled clinical studies. No overall differences in safety or effectiveness were observed between these subjects and younger adult subjects. Greater sensitivity of some older individuals cannot be ruled out.
Sleep-promoting drugs may cause confusion and over-sedation in the elderly. A starting dose of 3 mg is recommended in this population and evaluation prior to considering dose escalation is recommended [see Dosage and Administration (2.2)].
8.6. Use in Patients with Hepatic Impairment
Patients with hepatic impairment may display higher doxepin concentrations than healthy individuals. Initiate Silenor treatment with 3 mg in patients with hepatic impairment and monitor closely for adverse daytime effects. [see Clinical Pharmacology (12.5)]
8.7. Use in Patients with Sleep Apnea
Silenor has not been studied in patients with obstructive sleep apnea. Since hypnotics have the capacity to depress respiratory drive, precautions should be taken if Silenor is prescribed to patients with compromised respiratory function. In patients with severe sleep apnea, Silenor is ordinarily not recommended for use.
- 9. DRUG ABUSE AND DEPENDENCE
-
10. OVERDOSAGE
Doxepin is routinely administered for indications other than insomnia at doses 10- to 50-fold higher than the highest recommended dose of Silenor.
The signs and symptoms associated with doxepin use at doses several-fold higher than the maximum recommended dose (Excessive dose) of Silenor for the treatment of insomnia are described [see Overdosage (10.1)], as are signs and symptoms associated with higher multiples of the maximum recommended dose (Critical overdose) [see Overdosage (10.2)].
10.1. Signs and Symptoms of Excessive Doses
The following adverse effects have been associated with use of doxepin at doses higher than 6 mg.
Anticholinergic Effects: constipation and urinary retention.
Central Nervous System: disorientation, hallucinations, numbness, paresthesias, extrapyramidal symptoms, seizures, tardive dyskinesia.
Cardiovascular: hypotension.
Gastrointestinal: aphthous stomatitis, indigestion.
Endocrine: raised libido, testicular swelling, gynecomastia in males, enlargement of breasts and galactorrhea in the female, raising or lowering of blood sugar levels, and syndrome of inappropriate antidiuretic hormone secretion.
Other: tinnitus, weight gain, sweating, flushing, jaundice, alopecia, exacerbation of asthma, and hyperpyrexia (in association with chlorpromazine).
10.2. Signs and Symptoms of Critical Overdose
Manifestations of doxepin critical overdose include: cardiac dysrhythmias, severe hypotension, convulsions, and CNS depression including coma. Electrocardiogram changes, particularly in QRS axis or width, are clinically significant indicators of tricyclic compound toxicity. Other signs of overdose may include, but are not limited to: confusion, disturbed concentration, transient visual hallucinations, dilated pupils, agitation, hyperactive reflexes, stupor, drowsiness, muscle rigidity, vomiting, hypothermia, hyperpyrexia.
10.3. Recommended Management
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. In addition, the possibility of a multiple drug ingestion should be considered.
If an overdose is suspected, an ECG should be obtained and cardiac monitoring should be initiated immediately. The patient's airway should be protected, an intravenous line should be established, and gastric decontamination should be initiated. A minimum of six hours of observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is strongly advised. If signs of toxicity occur at any time during this period, extended monitoring is recommended. There are case reports of patients succumbing to fatal dysrhythmias late after overdose; these patients had clinical evidence of significant poisoning prior to death and most received inadequate gastrointestinal decontamination. Monitoring of plasma drug levels should not guide management of the patient.
Gastrointestinal Decontamination
All patients suspected of overdose should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by administration of activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage. Emesis is contraindicated.
Cardiovascular
A maximal limb-lead QRS duration of ≥0.10 seconds may be the best indication of the severity of an overdose. Serum alkalinization, using intravenous sodium bicarbonate should be used to maintain the serum pH in the range of 7.45 to 7.55 for patients with dysrhythmias and/or QRS widening. If the pH response is inadequate, hyperventilation may also be used. Concomitant use of hyperventilation and sodium bicarbonate should be done with extreme caution, with frequent pH monitoring. A pH >7.60 or a pCO2 <20 mm Hg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
In rare instances, hemoperfusion may be beneficial in acute refractory cardiovascular instability in patients with acute toxicity. However, hemodialysis, peritoneal dialysis, exchange transfusions, and forced diuresis generally have been reported as ineffective in treatment of tricyclic compound poisoning.
Central Nervous System
In patients with central nervous system depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines, or, if these are ineffective, other anticonvulsants (e.g., phenobarbital or phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in consultation with a poison control center.
-
11. DESCRIPTION
Silenor (doxepin) is available in 3 mg and 6 mg strength tablets for oral administration. Each tablet contains 3.39 mg or 6.78 mg doxepin hydrochloride, equivalent to 3 mg and 6mg of doxepin, respectively.
Chemically, doxepin hydrochloride is an (E) and (Z) geometric, isomeric mixture of 1 propanamine, 3-dibenz[b,e]oxepin-11(6H)ylidene-N,N-dimethyl-hydrochloride. It has the following structure:
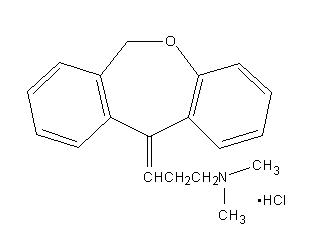
Doxepin hydrochloride is a white crystalline powder, with a slight amine-like odor, that is readily soluble in water. It has a molecular weight of 315.84 and molecular formula of C19 H21 NOHCl.
Each Silenor tablet includes the following inactive ingredients: microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate. The 3 mg tablet also contains FD&C Blue No.1. The 6 mg tablet also contains D&C Yellow No. 10 and FD&C Blue No. 1.
-
12. CLINICAL PHARMACOLOGY
12.1. Mechanism of Action
Doxepin binds with high affinity to the histamine H1 receptor (Ki < 1 nM) where it functions as an antagonist. The exact mechanism by which doxepin exerts its sleep maintenance effect is unknown but is believed due to its antagonism of the H1 receptor.
12.3. Pharmacokinetics
Absorption
The median time to peak concentrations (Tmax) of doxepin occurred at 3.5 hours postdose after oral administration of a 6 mg dose to fasted healthy subjects. Peak plasma concentrations (Cmax) of Silenor increased in approximately a dose-proportional manner for 3 mg and 6 mg doses. The AUC was increased by 41% and Cmax by 15% when 6 mg Silenor was administered with a high fat meal. Additionally, compared to the fasted state, Tmax was delayed by approximately 3 hours. Therefore, for faster onset and to minimize the potential for next day effects, it is recommended that Silenor not be taken within 3 hours of a meal [see Dosage and Administration (2.3)].
Distribution
Silenor is widely distributed throughout the body tissues. The mean apparent volume of distribution following a single 6 mg oral dose of Silenor to healthy subjects was 11,930 liters. Silenor is approximately 80% bound to plasma proteins.
Metabolism
Following oral administration, Silenor is extensively metabolized by oxidation and demethylation. The primary metabolite is N-desmethyldoxepin (nordoxepin).
The primary metabolite undergoes further biotransformation to glucuronide conjugates.
In vitro studies have shown that CYP2C19 and CYP2D6 are the major enzymes involved in doxepin metabolism, and that CYP1A2 and CYP2C9 are involved to a lesser extent.
Doxepin appears not to have inhibitory effects on human CYP enzymes at therapeutic concentrations. The potential of doxepin to induce metabolizing enzymes is not known. Doxepin is not a Pgp substrate.
12.4. Drug Interactions
Since doxepin is metabolized by CYP2C19 and CYP2D6, inhibitors of these CYP isozymes may increase the exposure of doxepin.
Cimetidine:
The effect of cimetidine, a non-specific inhibitor of CYP1A2, 2C19, 2D6, and 3A4, on Silenor plasma concentrations was evaluated in healthy subjects. When cimetidine 300 mg BID was co-administered with a single dose of Silenor 6 mg, there was approximately a 2-fold increase in Silenor Cmax and AUC compared to Silenor given alone. A maximum dose of doxepin in adults and elderly should be 3 mg, when doxepin is co-administered with cimetidine.
Sertraline:
The effect of sertraline HCl, a selective serotonin reuptake inhibitor, on doxepin plasma concentrations was evaluated in a daytime study conducted with 24 healthy subjects. Following co-administration of doxepin 6 mg with sertraline 50 mg (at steady-state), the doxepin mean AUC and Cmax estimates were approximately 21% and 32% higher, respectively, than those obtained following administration of doxepin alone. Psychomotor function as measured by the digit symbol substitution test and symbol copy test performance was decreased more at 2-4 hours post dosing for the combination of sertraline and doxepin as compared to doxepin alone, but subjective measures of alertness were comparable for the two treatments.
12.5. Special Populations
Renal Impairment
The effects of renal impairment on doxepin pharmacokinetics have not been studied. Because only small amounts of doxepin and nordoxepin are eliminated in the urine, renal impairment would not be expected to result in significantly altered doxepin concentrations.
-
13. NONCLINICAL TOXICOLOGY
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
No evidence of carcinogenic potential was observed when doxepin was administered orally to hemizygous Tg.rasH2 mice for 26 weeks at doses of 25, 50, 75 and 100 mg/kg/day.
Mutagenesis
Doxepin was negative in in vitro (bacterial reverse mutation, chromosomal aberration in human lymphocytes) and in vivo (rat micronucleus) assays.
Impairment of Fertility
When doxepin (10, 30 and 100 mg/kg/day) was orally administered to male and female rats prior to, during and after mating, adverse effects on fertility (increased copulatory interval and decreased corpora lutea, implantation, viable embryos and litter size) and sperm parameters (increased percentages of abnormal sperm and decreased sperm motility) were observed. The plasma exposures (AUC) for doxepin and nordoxepin at the no-effect dose for adverse effects on reproductive performance and fertility in rats (10 mg/kg/day) are less than those in humans at the maximum recommended human dose of 6 mg/day.
-
14. CLINICAL STUDIES
14.1. Controlled Clinical Trials
The efficacy of Silenor for improving sleep maintenance was supported by six randomized, double-blind studies up to 3 months in duration that included 1,423 subjects, 18 to 93 years of age, with chronic (N=858) or transient (N=565) insomnia. Silenor was evaluated at doses of 1 mg, 3 mg, and 6 mg relative to placebo in inpatient (sleep laboratory) and outpatient settings.
The primary efficacy measures for assessment of sleep maintenance were the objective and subjective time spent awake after sleep onset (respectively, objective Wake After Sleep Onset [WASO] and subjective WASO).
Subjects in studies of chronic insomnia were required to have at least a 3-month history of insomnia.
Chronic Insomnia
Adults
A randomized, double-blind, parallel-group study was conducted in adults (N = 221) with chronic insomnia. Silenor 3 mg and 6 mg was compared to placebo out to 30 days.
Silenor 3 mg and 6 mg were superior to placebo on objective WASO. Silenor 3 mg was superior to placebo on subjective WASO at night 1 only. Silenor 6 mg was superior to placebo on subjective WASO at night 1, and nominally superior at some later time points out to Day 30.
Elderly
Elderly subjects with chronic insomnia were assessed in two parallel-group studies.
The first randomized, double-blind study assessed Silenor 1 mg and 3 mg relative to placebo for 3 months in inpatient and outpatient settings in elderly subjects (N=240) with chronic insomnia. Silenor 3 mg was superior to placebo on objective WASO.
The second randomized, double-blind study assessed Silenor 6 mg relative to placebo for 4 weeks in an outpatient setting in elderly subjects (N=254) with chronic insomnia. On subjective WASO, Silenor 6 mg was superior to placebo.
Transient Insomnia
Healthy adult subjects (N=565) experiencing transient insomnia during the first night in a sleep laboratory were evaluated in a randomized, double-blind, parallel-group, single-dose study of Silenor 6 mg relative to placebo. Silenor 6 mg was superior to placebo on objective WASO and subjective WASO.
Withdrawal Effects
Potential withdrawal effects were assessed in a 35-day double blind study of adults with chronic insomnia who were randomized to placebo, Silenor 3 mg, or Silenor 6 mg. There was no indication of a withdrawal syndrome after discontinuation of Silenor treatment (3 mg or 6 mg), as measured by the Tyrer's Symptom Checklist. Discontinuation-period emergent nausea and vomiting occurred in 5% of subjects treated with 6 mg Silenor, versus 0% in 3 mg and placebo subjects.
-
16. HOW SUPPLIED/STORAGE AND HANDLING
16.1. How Supplied
Silenor 3 mg tablets are oval shaped, blue, identified with debossed markings of "3" on one side and "SP" on the other, and are supplied as:
NDC: 42847-103-30 Bottle of 30 NDC: 42847-103-10 Bottle of 100 NDC: 42847-103-50 Bottle of 500 NDC: 42847-103-03 Blister trade pack of 30 Silenor 6 mg tablets are oval shaped, green, identified with debossed markings of "6" on one side and "SP" on the other, and are supplied as:
NDC: 42847-106-30 Bottle of 30 NDC: 42847-106-10 Bottle of 100 NDC: 42847-106-50 Bottle of 500 NDC: 42847-106-03 Blister trade pack of 30 -
17. PATIENT COUNSELING INFORMATION
Prescribers or other healthcare professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with hypnotics, should counsel them in appropriate use, and should instruct them to read the accompanying Medication Guide [see Medication Guide (17.4)].
17.1. Sleep-driving and Other Complex Behaviors
There have been reports of people getting out of bed after taking a hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when a hypnotic is taken with alcohol or other central nervous system depressants [see Warnings and Precautions (5.2, 5.4) and Drug Interactions (7.3, 7.4)]. Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a hypnotic. As with "sleep-driving", patients usually do not remember these events.
In addition, patients should be advised to report all concomitant medications to the prescriber. Patients should be instructed to report events such as "sleep-driving" and other complex behaviors immediately to the prescriber.
17.2. Suicide risk and Worsening of Depression:
Patients, their families, and their caregivers should be encouraged to be alert to worsening of depression, including suicidal thoughts and actions. Such symptoms should be reported to the patient's prescriber or health professional.
17.3. Administration Instructions
Patients should be counseled to take Silenor within 30 minutes of bedtime and should confine their activities to those necessary to prepare for bed. Silenor tablets should not be taken with or immediately after a meal [see Dosage and Administration (2.3)]. Advise patients NOT to take Silenor when drinking alcohol [see Warnings and Precautions (5.2, 5.4) and Drug Interactions (7.3)].
-
MEDICATION GUIDE
MEDICATION GUIDE
SILENOR® (SI-leh-nor) Tablets
(doxepin)Read this Medication Guide before you start taking SILENOR and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
What is the most important information I should know about SILENOR?
After taking SILENOR, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with SILENOR. Reported activities include:
- driving a car ("sleep-driving")
- making and eating food
- talking on the phone
- having sex
- sleep-walking
Call your healthcare provider right away if you find out that you have done any of the above activities after taking SILENOR.
Important:
- 1.
Take SILENOR exactly as prescribed
- Do not take more SILENOR than prescribed.
- Take SILENOR 30 minutes before bedtime. After taking SILENOR, you should only do activities needed to get ready for bed.
- 2. Do not take SILENOR:
- with alcohol
- if you take other medicines that can make you sleepy. Talk to your healthcare provider about all of your medicines. Your healthcare provider will tell you if you can take SILENOR with your other medicines
- if you cannot get a full night of sleep before you must be active again
What is SILENOR?
SILENOR is a hypnotic (sleep) medicine that is used to treat people who have trouble staying asleep.
Do not take SILENOR if you:
- take a monoamine oxidase inhibitor (MAOI) medicine or have taken an MAOI in the last 14 days (2 weeks). Ask your healthcare provider if you are not sure if your medicine is an MAOI.
- have an eye problem called narrow angle glaucoma that is not being treated
- have trouble urinating
- are allergic to any of the ingredients in SILENOR. See the end of this Medication Guide for a complete list of ingredients in SILENOR.
Talk to your healthcare provider before taking this medicine if you have any of these conditions.
It is not known if SILENOR is safe and effective in children.
What should I tell my healthcare provider before taking SILENOR?
Before you take SILENOR, tell your healthcare provider if you:
- See "Who should not take Silenor?"
- have a history of depression, mental illness, or suicidal thoughts
- have severe sleep apnea
- have kidney or liver problems
- have a history of drug or alcohol abuse or addiction
- have a history of glaucoma or urinary retention
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if SILENOR will harm your unborn baby. Talk to your healthcare provider if you are pregnant or plan to become pregnant.
- are breast-feeding or plan to breast-feed. SILENOR can pass into your milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby if you take SILENOR. You should not breast-feed while taking SILENOR.
Tell your doctor about all of the medicines you take including prescription and nonprescription medicines, vitamins and herbal supplements.
SILENOR and other medicines may affect each other causing side effects. SILENOR may affect the way other medicines work, and other medicines may affects how SILENOR works. Especially tell your healthcare provider if you take:
- a monoamine oxidase inhibitor (MAOI). See "Who should not take SILENOR?"
- cimetidine (Tagamet) or other medicines that can affect certain liver enzymes
- certain allergy medicines (antihistamines) or other medicines that can make you sleepy or affect your breathing
- the diabetes medicine tolazamide
Ask your doctor or pharmacist if you are not sure if your medicine is one that is listed above.
Know the medicines you take. Keep a list of your medicines with you to show your doctor and pharmacist each time you get a new medicine.
How should I take SILENOR?
- Take SILENOR exactly as your healthcare provider tells you to take it.
- Your doctor will tell you how many SILENOR to take and when to take them.
- Your doctor may change your dose if needed.
- Take SILENOR within 30 minutes of bedtime. After taking SILENOR, you should confine your activities to those necessary to prepare for bed.
- Do not take SILENOR within 3 hours of a meal. Silenor may not work as well, or may make you sleepy the next day if taken with or right after a meal.
- Do not take SILENOR unless you are able to get a full night of sleep before you must be active again.
- Call your doctor if your sleep problems get worse or do not get better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- If you take too much SILENOR, call your doctor or get medical help right away.
What should I avoid while taking SILENOR?
- You should not drink alcohol while taking SILENOR. Alcohol can increase your chances of getting serious side effects with SILENOR.
- You should not drive, operate heavy machinery, or do other dangerous activities after SILENOR.
You may still feel drowsy the next day after taking SILENOR. Do not drive or do other dangerous activities after taking SILENOR until you feel fully awake.
What are the possible side effects of SILENOR?
SILENOR can cause serious side effects including:
The most common side effect of SILENOR is drowsiness or tiredness.
Tell your healthcare provider if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of SILENOR. For more information ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to the FDA at 1-800-FDA-1088.
How should I store SILENOR?
- Store SILENOR between 68° and 77° F (20° to 25°C).
- Keep SILENOR in a tightly closed container, and away from light. Safely throw away medicine that is out of date or no longer needed.
- Keep SILENOR and all medicines out of the reach of children.
General Information about SILENOR
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SILENOR for a condition for which it was not prescribed. Do not share SILENOR with other people, even if you think they have the same symptoms that you have. It may harm them.
This Medication Guide summarizes the most important information about SILENOR. If you would like more information, talk with your healthcare provider. You can ask your healthcare provider or pharmacist for information about SILENOR that is written for healthcare professionals.
For more information, contact Currax™ Pharmaceuticals LLC at 1-800-793-2145.
What are the ingredients in SILENOR?
Active Ingredient: doxepin hydrochloride
Inactive Ingredients: Microcrystalline cellulose, colloidal silicon dioxide, and magnesium stearate. The 3 mg tablet also contains FD&C Blue No. 1. The 6 mg tablet also contains FD&C Yellow No. 10 and FD&C Blue No. 1.
Distributed by:
Currax™ Pharmaceuticals LLC
Morristown, NJ 07960 USAThis Medication Guide has been approved by the U.S. Food and Drug Administration.
SIL-LC087.00 08/19
-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 3 mg Tablet Bottle Label
3mg
30 tablets
Rx Onlysilenor®
doxepin tabletsNDC: 42847-103-30

-
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 6 mg Tablet Bottle Label
6mg
30 tablets
Rx Onlysilenor®
doxepin tabletsNDC: 42847-106-30

-
INGREDIENTS AND APPEARANCE
SILENOR
doxepin hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42847-103 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength doxepin hydrochloride (UNII: 3U9A0FE9N5) (doxepin - UNII:5ASJ6HUZ7D) doxepin 3 mg Inactive Ingredients Ingredient Name Strength Microcrystalline Cellulose (UNII: OP1R32D61U) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) FD&C Blue No. 1 (UNII: H3R47K3TBD) Product Characteristics Color BLUE Score no score Shape OVAL Size 10mm Flavor Imprint Code 3;SP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42847-103-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2010 2 NDC: 42847-103-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2010 3 NDC: 42847-103-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2010 4 NDC: 42847-103-03 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022036 08/01/2010 SILENOR
doxepin hydrochloride tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42847-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength doxepin hydrochloride (UNII: 3U9A0FE9N5) (doxepin - UNII:5ASJ6HUZ7D) doxepin 6 mg Inactive Ingredients Ingredient Name Strength Microcrystalline Cellulose (UNII: OP1R32D61U) silicon dioxide (UNII: ETJ7Z6XBU4) magnesium stearate (UNII: 70097M6I30) D&C Yellow No. 10 (UNII: 35SW5USQ3G) FD&C Blue No. 1 (UNII: H3R47K3TBD) Product Characteristics Color GREEN Score no score Shape OVAL Size 10mm Flavor Imprint Code 6;SP Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42847-106-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2010 2 NDC: 42847-106-10 100 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2010 3 NDC: 42847-106-50 500 in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2010 4 NDC: 42847-106-03 30 in 1 BLISTER PACK; Type 0: Not a Combination Product 08/01/2010 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA022036 08/01/2010 Labeler - Currax Pharmaceuticals LLC (117055730) Establishment Name Address ID/FEI Business Operations Patheon Pharmaceuticals, Inc 005286822 MANUFACTURE(42847-103, 42847-106) Establishment Name Address ID/FEI Business Operations Mylan Pharmaceuticals 059295980 MANUFACTURE(42847-103, 42847-106) , PACK(42847-103, 42847-106) Establishment Name Address ID/FEI Business Operations PCI 078525133 PACK(42847-103, 42847-106) Establishment Name Address ID/FEI Business Operations Plantex Ltd 600023907 API MANUFACTURE(42847-103, 42847-106)
Trademark Results [Silenor]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SILENOR 78640504 3477986 Live/Registered |
CURRAX PHARMACEUTICALS LLC 2005-05-31 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.