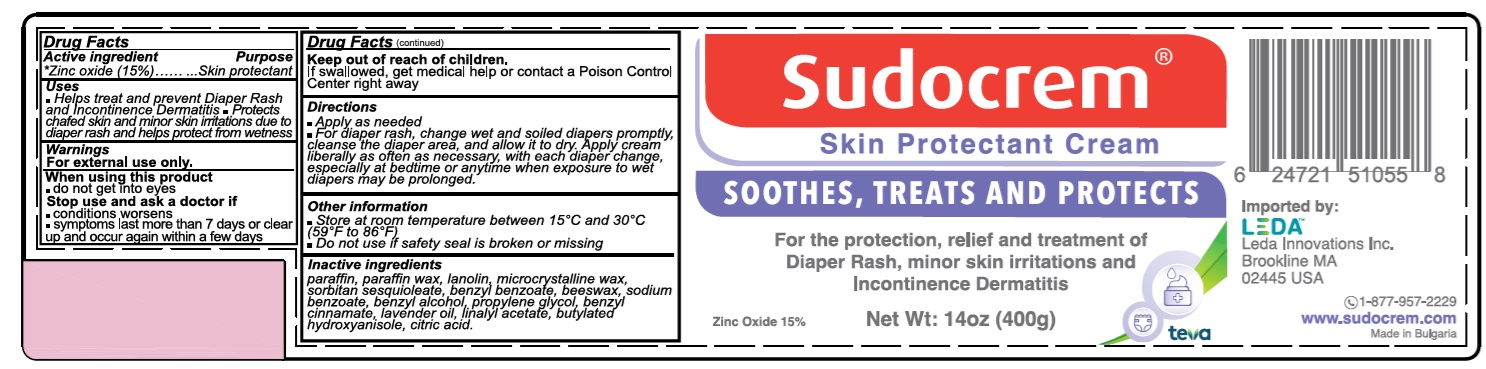
SUDOCREM SKIN PROTECTANT CREAM
SUDOCREM SKIN PROTECTANT by
Drug Labeling and Warnings
SUDOCREM SKIN PROTECTANT by is a Otc medication manufactured, distributed, or labeled by LEDA INNOVATIONS USA, INC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SUDOCREM SKIN PROTECTANT- zinc oxide cream
LEDA INNOVATIONS USA, INC.
----------
SUDOCREM SKIN PROTECTANT CREAM
Uses
- Helps treat and prevent Diaper Rash and Incontinence Dermatitis
- Protects chafed skin and minor skin irritations due to diaper rash and helps protect from wetness
Warnings
For external use only.
Directions
- Apply as needed
- For diaper rash, change wet and soiled diapers promptly, cleanse the diaper area, and allow it to dry. Apply cream liberally as often as necessary, with each diaper change, especially at bedtime or anytime when exposure to wet diapers may be prolonged.
Other information
- Store at room temperature between 15C and 30C (59F to 86F)
- Do not use if safety seal is broken or missing
| SUDOCREM SKIN PROTECTANT
zinc oxide cream |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - LEDA INNOVATIONS USA, INC. (779937036) |
Revised: 8/2024
Document Id: 20d31212-4426-85c4-e063-6394a90a19ff
Set id: 1f0a67cb-cbd1-2314-e063-6394a90a09e4
Version: 2
Effective Time: 20240829