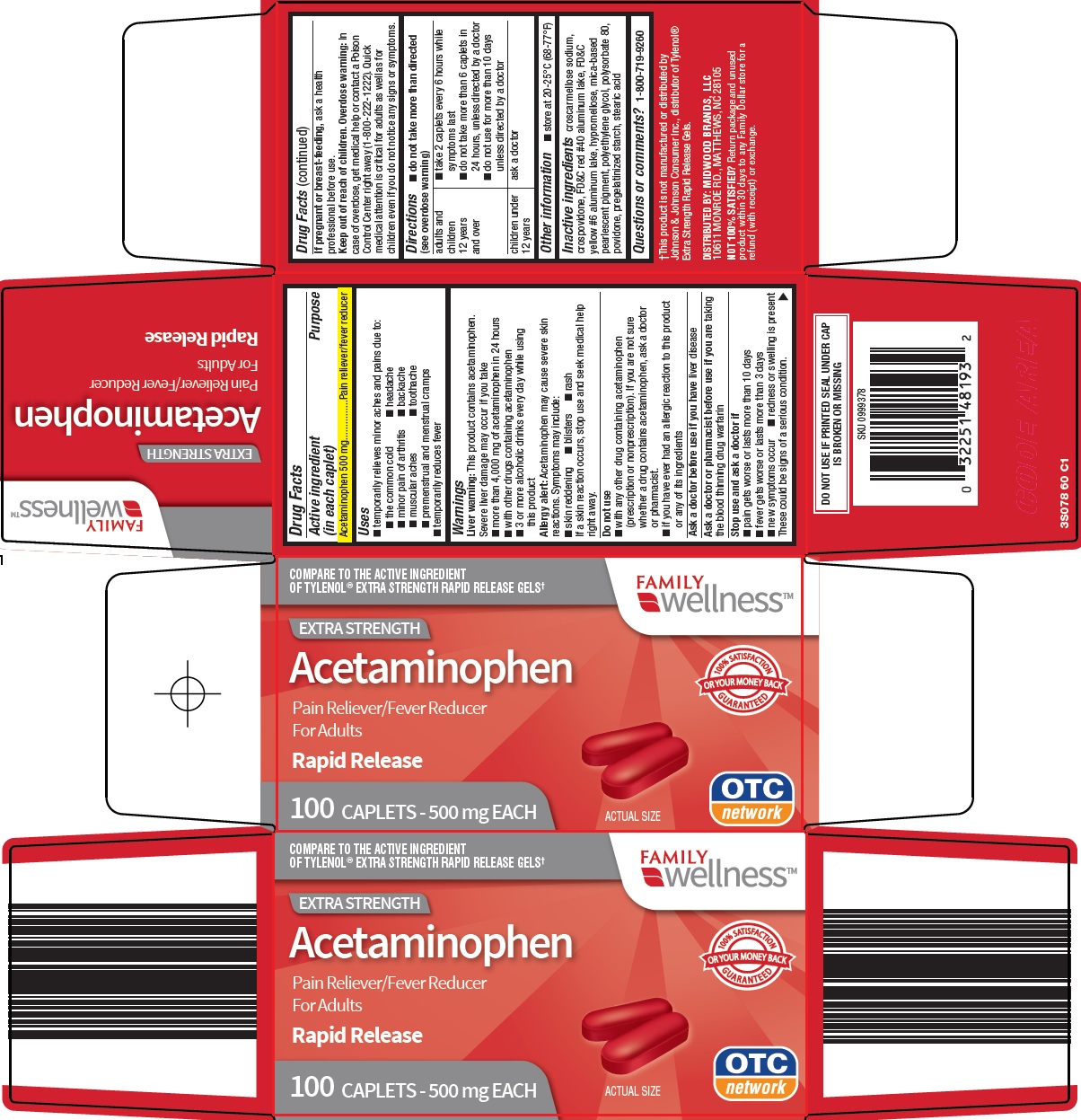
Family Dollar Services, Inc. Acetaminophen Drug Facts
family wellness acetaminophen by
Drug Labeling and Warnings
family wellness acetaminophen by is a Otc medication manufactured, distributed, or labeled by Family Dollar Services Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FAMILY WELLNESS ACETAMINOPHEN- acetaminophen tablet, film coated
Family Dollar Services Inc
----------
Family Dollar Services, Inc. Acetaminophen Drug Facts
Uses
- temporarily relieves minor aches and pains due to:
- headache
- muscular aches
- backache
- minor pain of arthritis
- the common cold
- toothache
- premenstrual and menstrual cramps
- temporarily reduces fever
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- more than 4,000 mg of acetaminophen in 24 hours
- with other drugs containing acetaminophen
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- skin reddening
- blisters
- rash
If a skin reaction occurs, stop use and seek medical help right away.
Do not use
- with any other drug containing acetaminophen (prescription or nonprescription). If you are not sure whether a drug contains acetaminophen, ask a doctor or pharmacist.
- if you have ever had an allergic reaction to this product or any of its ingredients
Directions
- do not take more than directed (see overdose warning)
|
adults and children 12 years and over |
|
|
children under 12 years |
ask a doctor |
| FAMILY WELLNESS ACETAMINOPHEN
acetaminophen tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Family Dollar Services Inc (024472631) |
Revised: 11/2024
Document Id: 3fa05974-f977-4ed4-8d86-89df1adb4aee
Set id: 2025c032-02f2-41be-9c14-636175aefa8b
Version: 2
Effective Time: 20241108