ZINGIBER- pyridoxine, folic acid, calcium, and ginger tablet tablet
Zingiber by
Drug Labeling and Warnings
Zingiber by is a Prescription medication manufactured, distributed, or labeled by PruGen, Inc. Pharmaceuticals, NuLab, Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
WARNINGS AND PRECAUTIONS
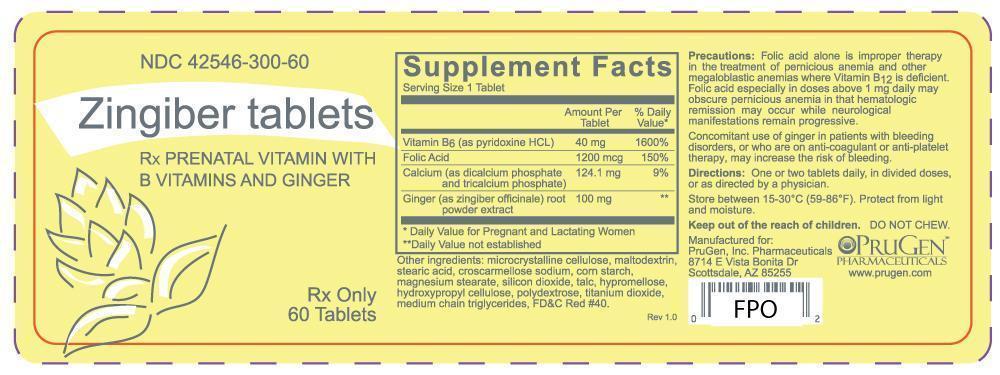
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient. Folic acid especially in doses above 1 mg daily may obscure pernicious anemia in that hematologic remission may occur while neurological manifestations remain progressive.
Concomitant use of ginger in patients with bleeding disorders, or who are on anti-coagulant or anti-platelet therapy, may increase the risk of bleeding.
-
DESCRIPTION
Each pink tablet contains:
Vitamin B6 (pyridoxine hydrochloride, USP) 40 mg
Folic Acid, USP 1.2 mg
Calcium (as dicalcium phosphate and tricalcium phosphate) 124.1 mg
Ginger (zingiber officinale) root powder extract 100 mg
Other ingredients: microcrystalline cellulose, maltodextrin, stearic acid, croscarmellose sodium, corn starch, magnesium stearate, silicon dioxide, talc, hypromellose, hydroxypropyl cellulose, polydextrose, titanium dioxide, medium chain triglycerides, FD&C Red #40.
- DOSAGE & ADMINISTRATION
- HOW SUPPLIED
-
PRINCIPAL DISPLAY PANEL
NDC: 42546-300-60
Zingiber Tablets
Rx prenatal vitamin with B vitamins and ginger
Rx Only
60 tablets
-
INGREDIENTS AND APPEARANCE
ZINGIBER
pyridoxine, folic acid, calcium, and ginger tablet tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42546-300 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength PYRIDOXINE (UNII: KV2JZ1BI6Z) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 40 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1.2 mg CALCIUM (UNII: SY7Q814VUP) (CALCIUM - UNII:SY7Q814VUP) CALCIUM 124.1 mg GINGER (UNII: C5529G5JPQ) (GINGER - UNII:C5529G5JPQ) GINGER 100 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) MALTODEXTRIN (UNII: 7CVR7L4A2D) STEARIC ACID (UNII: 4ELV7Z65AP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STARCH, CORN (UNII: O8232NY3SJ) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSES (UNII: 3NXW29V3WO) HYDROXYPROPYL CELLULOSE (UNII: RFW2ET671P) POLYDEXTROSE (UNII: VH2XOU12IE) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color pink Score no score Shape OVAL Size 24mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42546-300-60 60 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 02/01/2013 Labeler - PruGen, Inc. Pharmaceuticals (929922750) Establishment Name Address ID/FEI Business Operations NuLab, Inc 102098324 manufacture(42546-300)
Trademark Results [Zingiber]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 ZINGIBER 88309767 5844770 Live/Registered |
Shenzhenshi zhongbo gaoxinkeji youxiangongsi 2019-02-21 |
 ZINGIBER 74298633 not registered Dead/Abandoned |
Bioenergy Nutrient's Inc. 1992-07-27 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.