IBUPROFEN 100- ibuprofen tablet, coated IBUPROFEN 250- ibuprofen tablet, coated
IBUPROFEN 250 by
Drug Labeling and Warnings
IBUPROFEN 250 by is a Otc medication manufactured, distributed, or labeled by JHK Inc dba American Safety & First Aid. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient (In Each Tablets)
- Purpose
- Use
-
Warnings
Allergy Alert
Ibuprofen may cause a severe allergic reaction, especially in people allergic to aspirin. Symptoms may include:
- hives
- asthma (wheezing)
- facial swelling
- shock
- skin reddening
- rash
- blisters
- If an allergic reaction occurs, stop use and seek medical help right away.
Stomach bleeding warning
This product contains a nonsteroidal anti-inflammatory drug (NSAID), which may cause severe stomach bleeding. The chance is higher if you:
- are age 60 or older
- have had stomach ulcers or bleeding problems
- take a blood thinning (anticoagulant) or steroid drug
- take other drugs containing prescription or nonprescription NSAIDs (aspirin, ibuprofen, naproxen, or others)
- have 3 or more alcoholic
- drinks every day while using this product
- take more or for a longer time than directed
Do not use
- if you ever had an allergic reaction to any other pain reliever/fever reducer
- right before or after heart surgery
Ask a doctor before use if
- stomach bleeding warning applies to you
- you have a history of stomach problems, such as heartburn
- you have high blood pressure, heart disease, liver cirrhosis, or kidney disease
- you are taking a diuretic
Ask a doctor or pharmacist before use if you are
- taking any other drug containing an NSAID (prescription or nonprescription)
- taking a blood thinning (anticoagulant) drug
- under a doctor's care for any serious condition
- taking any other drug
When using this product
- take with food or milk if stomach upset occurs
- long term continuous use may increase the risk of heart attack or stroke
Stop use and ask a doctor if you experience any of the following signs of stomach bleeding:
- feel faint
- vomit blood
- have bloody or black stools
- have stomach pain that does not get better
- pain gets worse or lasts more than 10 days
- fever gets worse or lasts more than 3 days
- redness or swelling is present in the painful area
- any new symptoms appear
-
Directions
- do not take more than directed
- the smallest effective dose should be used
- do not take longer than 10 days, unless directed by a doctor (see Warnings)
Adults: 1 tablet every 4 to 6 hours while symptoms occur; if pain or fever does not respond to 1 tablet, 2 tablets may be used, but do not exceed 6 tablets in 24 hours unless directed by a doctor.
Children under 12 years: Ask a doctor.
- Other Information
-
Inactive Ingredients
crosscarmellose sodium1, FD& C Blue #21, FD& C red #401, FD& C Yellow #61, hypromellose1, iron oxide red1, lactose monohydrate1, magnesium stearate1, maltodextrin1, medium chain triglycerides1, microcrystalline cellulose1, polydextrose1, polyethylene glycol1, polyvinyl alcohol1, povidone (K-30)1, silicon dioxide, sodium starch glycolate1, starch, stearic acid1, talc1, titanium dioxide, triacetin1,
- 1 contains one or more of these ingredients
- Questions?
-
PRINCIPAL DISPLAY PANEL - 100 Tablet Packet Box
This product is not manufactured or distributed by
Pfizer Consumer Healthcare
Owner of the registered trademark Advil®.Product #1102
Ibuprofen
Pain Reliever/Fever Reducer
Active ingredient:
Ibuprofen- Temporary relief from pain, fever and inflammation
- Soothes muscles aches and minor arthritis pain
- Non-drowsy, aspirin-free formula
These quality comfort tablets are formulated and packaged for use in the
workplace and meet ANSI Z308.1-2015 standards. They are sealed in
tamper-evident packets for safety and convenience.100 Tablets 2 Tablets Per Packet

-
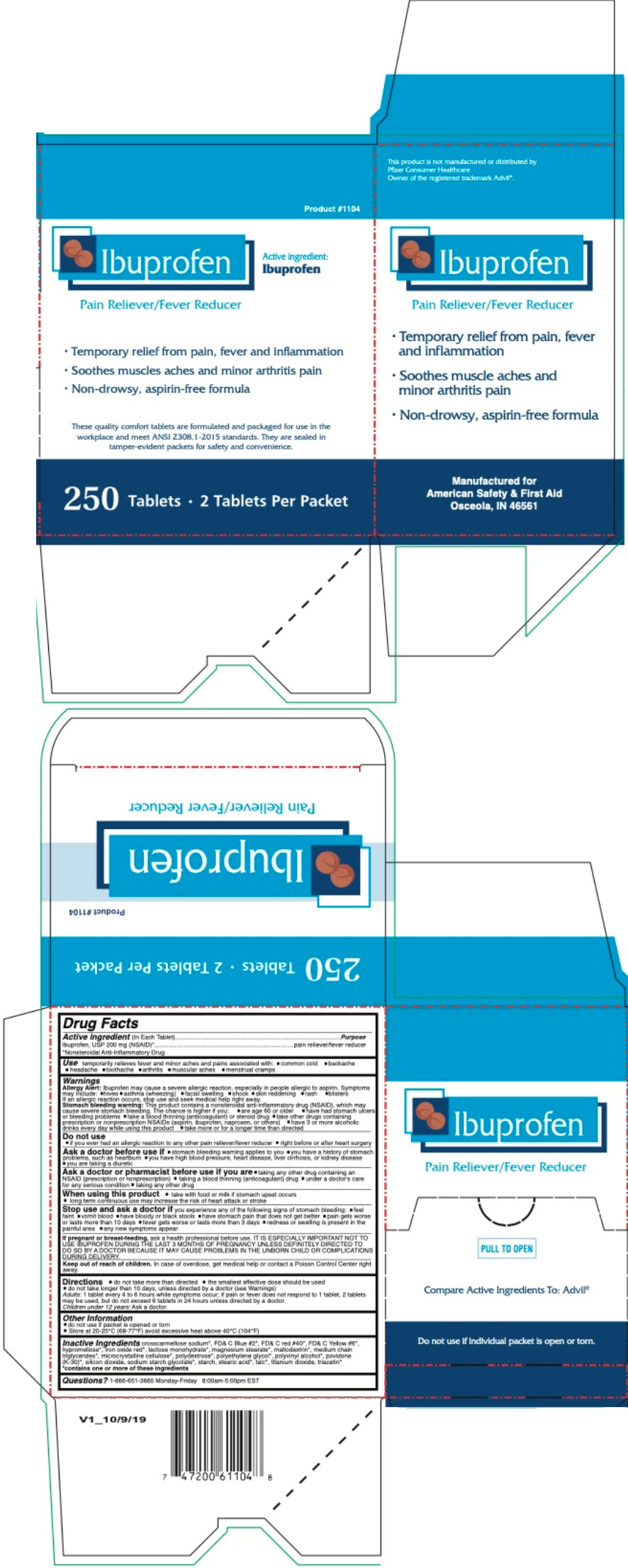
PRINCIPAL DISPLAY PANEL - 250 Tablet Packet Box
Product #1104
Ibuprofen
Pain Reliever/Fever Reducer
Active ingredient:
Ibuprofen- Temporary relief from pain, fever and inflammation
- Soothes muscles aches and minor arthritis pain
- Non-drowsy, aspirin-free formula
These quality comfort tablets are formulated and packaged for use in the
workplace and meet ANSI Z308.1-2015 standards. They are sealed in
tamper-evident packets for safety and convenience.250 Tablets 2 Tablets Per Packet
-
INGREDIENTS AND APPEARANCE
IBUPROFEN 100
ibuprofen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73598-1102 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code G2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73598-1102-1 50 in 1 BOX 02/02/2000 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072096 02/02/2000 IBUPROFEN 250
ibuprofen tablet, coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73598-1104 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength IBUPROFEN (UNII: WK2XYI10QM) (IBUPROFEN - UNII:WK2XYI10QM) IBUPROFEN 200 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) FERRIC OXIDE RED (UNII: 1K09F3G675) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) MALTODEXTRIN (UNII: 7CVR7L4A2D) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) POVIDONE K30 (UNII: U725QWY32X) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) SODIUM STARCH GLYCOLATE TYPE A (UNII: H8AV0SQX4D) STARCH, CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Product Characteristics Color BROWN Score 2 pieces Shape ROUND Size 10mm Flavor Imprint Code G2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73598-1104-1 125 in 1 BOX 02/02/2000 1 2 in 1 PACKET; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA072096 02/02/2000 Labeler - JHK Inc dba American Safety & First Aid (867236309)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.