NICARDIPINE HYDROCHLORIDE capsule
Nicardipine Hydrochloride by
Drug Labeling and Warnings
Nicardipine Hydrochloride by is a Prescription medication manufactured, distributed, or labeled by Carilion Materials Management. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Nicardipine hydrochloride capsules for oral administration each contain 20 mg or 30 mg of nicardipine hydrochloride. Nicardipine hydrochloride is a calcium ion influx inhibitor (slow channel blocker or calcium channel blocker).
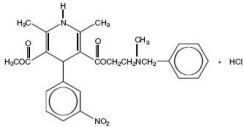
Nicardipine hydrochloride is a dihydropyridine structure with the IUPAC (International Union of Pure and Applied Chemistry) chemical name 2-(Benzylmethylamino)ethyl methyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate monohydrochloride, and it has the following structural formula:
Nicardipine hydrochloride, USP is a greenish-yellow, odorless, crystalline powder that melts at about 169°C. It is freely soluble in chloroform, methanol, and glacial acetic acid, sparingly soluble in anhydrous ethanol, slightly soluble in n-butanol, water, 0.01 M potassium dihydrogen phosphate, acetone, and dioxane, very slightly soluble in ethyl acetate, and practically insoluble in benzene, ether and hexane. It has a molecular weight of 515.99.
Each capsule, for oral administration, contains 20 mg or 30 mg of nicardipine hydrochloride, USP. In addition, each capsule contains the following inactive ingredients: colloidal silicon dioxide, gelatin, magnesium stearate, pharmaceutical glaze, pregelatinized starch, propylene glycol, silicon dioxide, sodium lauryl sulfate, synthetic black iron oxide, FD&C Blue No.2 Aluminum Lake, FD&C Red No.40 Aluminum Lake, FD&C Blue No.1 Aluminum Lake, and D&C Yellow No.10 Aluminum Lake. The colorants used in the 20 mg capsules are titanium dioxide, D&C Yellow No.10, and FD&C Green No.3. The colorants used in the 30 mg capsules are titanium dioxide, yellow iron oxide, and FD&C Green No.3.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Nicardipine is a calcium entry blocker (slow channel blocker or calcium ion antagonist) which inhibits the transmembrane influx of calcium ions into cardiac muscle and smooth muscle without changing serum calcium concentrations. The contractile processes of cardiac muscle and vascular smooth muscle are dependent upon the movement of extracellular calcium ions into these cells through specific ion channels. The effects of nicardipine are more selective to vascular smooth muscle than cardiac muscle. In animal models, nicardipine produces relaxation of coronary vascular smooth muscle at drug levels which cause little or no negative inotropic effect.
Pharmacokinetics and Metabolism
Nicardipine is completely absorbed following oral doses administered as capsules. Plasma levels are detectable as early as 20 minutes following an oral dose and maximal plasma levels are observed within 30 minutes to 2 hours (mean Tmax = 1 hour). While nicardipine is completely absorbed, it is subject to saturable first pass metabolism and the systemic bioavailability is about 35% following a 30 mg oral dose at steady-state.
When nicardipine hydrochloride was administered one (1) or three (3) hours after a high fat meal, the mean Cmax and mean AUC were lower (20% to 30%) than when nicardipine hydrochloride was given to fasting subjects. These decreases in plasma levels observed following a meal may be significant but the clinical trials establishing the efficacy and safety of nicardipine hydrochloride were done in patients without regard to the timing of meals. Thus the results of these trials reflect the effects of meal-induced variability.
The pharmacokinetics of nicardipine are nonlinear due to saturable hepatic first-pass metabolism. Following oral administration, increasing doses result in a disproportionate increase in plasma levels. Steady-state Cmax values following 20 mg, 30 mg, and 40 mg doses every 8 hours averaged 36, 88, and 133 ng/mL, respectively. Hence, increasing the dose from 20 mg to 30 mg every 8 hours more than doubled Cmax and increasing the dose from 20 mg to 40 mg every 8 hours increased Cmax more than 3-fold. A similar disproportionate increase in AUC with dose was observed. Considerable intersubject variability in plasma levels was also observed.
Post-absorption kinetics of nicardipine are also non-linear, although there is a reproducible terminal plasma half-life that averaged 8.6 hours following 30 mg and 40 mg doses at steady-state (TID). The terminal half-life represents the elimination of less than 5% of the absorbed drug (measured by plasma concentrations). Elimination over the first 8 hours after dosing is much faster with a half-life of 2 to 4 hours. Steady-state plasma levels are achieved after 2 to 3 days of TID dosing (every 8 hours) and are 2-fold higher than after a single dose.
Nicardipine is highly protein bound ( > 95%) in human plasma over a wide concentration range.
Nicardipine is metabolized extensively by the liver; less than 1% of intact drug is detected in the urine. Following a radioactive oral dose in solution, 60% of the radioactivity was recovered in the urine and 35% in feces. Most of the dose (over 90%) was recovered within 48 hours of dosing. Nicardipine does not induce its own metabolism and does not induce hepatic microsomal enzymes.
Nicardipine plasma levels were higher in patients with mild renal impairment (baseline serum creatinine concentration ranged from 1.2 to 5.5 mg/dL) than in normal subjects. After 30 mg nicardipine hydrochloride TID at steady-state, Cmax and AUC were approximately 2-fold higher in these patients.
Because nicardipine is extensively metabolized by the liver, the plasma levels of the drug are influenced by changes in hepatic function. Nicardipine plasma levels were higher in patients with severe liver disease (hepatic cirrhosis confirmed by liver biopsy or presence of endoscopically-confirmed esophageal varices) than in normal subjects. After 20 mg nicardipine hydrochloride BID at steady-state, Cmax and AUC were 1.8- and 4-fold higher, and the terminal half-life was prolonged to 19 hours in these patients.
Geriatric Pharmacokinetics
The steady-state pharmacokinetics of nicardipine in elderly hypertensive patients (≥ 65 years) are similar to those obtained in young normal adults. After one week of nicardipine hydrochloride dosing at 20 mg 3 times a day, the Cmax, Tmax, AUC, terminal plasma half-life, and the extent of protein binding of nicardipine observed in healthy elderly hypertensive patients did not differ significantly from those observed in young normal volunteers.
Hemodynamics
In man, nicardipine produces a significant decrease in systemic vascular resistance. The degree of vasodilation and the resultant hypotensive effects are more prominent in hypertensive patients. In hypertensive patients, nicardipine reduces the blood pressure at rest and during isometric and dynamic exercise. In normotensive patients, a small decrease of about 9 mmHg in systolic and 7 mmHg in diastolic blood pressure may accompany this fall in peripheral resistance. An increase in heart rate may occur in response to the vasodilation and decrease in blood pressure, and in a few patients this heart rate increase may be pronounced. In clinical studies mean heart rate at time of peak plasma levels was usually increased by 5 to 10 beats per minute compared to placebo, with the greater increases at higher doses, while there was no difference from placebo at the end of the dosing interval. Hemodynamic studies following intravenous dosing in patients with coronary artery disease and normal or moderately abnormal left ventricular function have shown significant increases in ejection fraction and cardiac output with no significant change, or a small decrease, in left ventricular end-diastolic pressure (LVEDP). Although there is evidence that nicardipine increases coronary blood flow, there is no evidence that this property plays any role in its effectiveness in stable angina. In patients with coronary artery disease, intra-coronary administration of nicardipine caused no direct myocardial depression. Nicardipine does, however, have a negative inotropic effect in some patients with severe left ventricular dysfunction and could, in patients with very impaired function, lead to worsened failure.
"Coronary Steal", the detrimental redistribution of coronary blood flow in patients with coronary artery disease (diversion of blood from under perfused areas toward better perfused areas), has not been observed during nicardipine treatment. On the contrary, nicardipine has been shown to improve systolic shortening in normal and hypokinetic segments of myocardial muscle, and radio-nuclide angiography has confirmed that wall motion remained improved during an increase in oxygen demand. Nonetheless, occasional patients have developed increased angina upon receiving nicardipine. Whether this represents steal in those patients, or is the result of increased heart rate and decreased diastolic pressure, is not clear.
In patients with coronary artery disease nicardipine improves L.V. diastolic distensibility during the early filling phase, probably due to a faster rate of myocardial relaxation in previously under perfused areas. There is little or no effect on normal myocardium, suggesting the improvement is mainly by indirect mechanisms such as afterload reduction, and reduced ischemia. Nicardipine has no negative effect on myocardial relaxation at therapeutic doses. The clinical consequences of these properties are as yet undemonstrated.
Electrophysiologic Effects
In general, no detrimental effects on the cardiac conduction system were seen with the use of nicardipine hydrochloride.
Nicardipine increased the heart rate when given intravenously during acute electrophysiologic studies, and prolonged the corrected QT interval to a minor degree. The sinus node recovery times and SA conduction times were not affected by the drug. The PA, AH, and HV intervals1 and the functional and effective refractory periods of the atrium were not prolonged by nicardipine and the relative and effective refractory periods of the His-Purkinje system were slightly shortened after intravenous nicardipine.
- 1 PA = conduction time from high to low right atrium, AH = conduction time from low right atrium to His bundle deflection, or AV nodal conduction time, HV = conduction time through the His bundle and the bundle branch-Purkinje system.
Renal Function
There is a transient increase in electrolyte excretion, including sodium. Nicardipine does not cause generalized fluid retention, as measured by weight changes, although 7% to 8% of the patients experience pedal edema.
Effects In Angina Pectoris
In controlled clinical trials of up to 12-weeks duration in patients with chronic stable angina, nicardipine increased exercise tolerance and reduced nitroglycerin consumption and the frequency of anginal attacks. The antianginal efficacy of nicardipine hydrochloride (20 mg to 40 mg) has been demonstrated in four placebo-controlled studies involving 258 patients with chronic stable angina. In exercise tolerance testing, nicardipine significantly increased time to angina, total exercise duration and time to 1 mm ST segment depression. Included among these four studies was a dose-definition study in which dose related improvements in exercise tolerance at one and four hours post-dosing and reduced frequency of anginal attacks were seen at doses of 10 mg, 20 mg and 30 mg TID. Effectiveness at 10 mg TID was, however, marginal. In a fifth placebo-controlled study, the antianginal efficacy of nicardipine was demonstrated at 8 hours post-dose (trough). The sustained efficacy of nicardipine has been demonstrated over long-term dosing. Blood pressure fell in patients with angina by about 10/8 mmHg at peak blood levels and was little different from placebo at trough blood levels.
Effects In Hypertension
Nicardipine produced dose related decreases in both systolic and diastolic blood pressure in clinical trials. The antihypertensive efficacy of nicardipine administered 3 times daily has been demonstrated in three placebo-controlled studies involving 517 patients with mild to moderate hypertension. The blood pressure responses in the three studies were statistically significant from placebo at peak (1 hour post-dosing) and trough (8 hours post-dosing) although it is apparent that well over half of the antihypertensive effect is lost by the end of the dosing interval. The results from placebo controlled studies of nicardipine given 3 times daily are shown in the following table:
SYSTOLIC BP (mmHg)
Dose
Number of Patients
Mean Peak Response
Mean Trough Response
Trough/Peak
20 mg
50
52-10.3
-17.6-4.9
-7.948%
45%30 mg
45
44-14.5
-14.6-7.2
-7.550%
51%40 mg
50
38-16.3
-15.99.5
658%
38%DIASTOLIC BP (mmHg)
Dose
Number of Patients
Mean Peak Response
Mean Trough Response
Trough/Peak
20 mg
50
52-10.6
-9-4.6
-2.943%
32%30 mg
45
44-12.8
-14.2-4.9
-4.338%
30%40 mg
50
38-15.4
-14.8-5.9
-3.738%
25%The responses are shown as differences from the concurrent placebo control group. The large changes between peak and trough effects were not accompanied by observed side effects at peak response times. In a study using 24 hour intra-arterial blood pressure monitoring, the circadian variation in blood pressure remained unaltered, but the systolic and diastolic blood pressures were reduced throughout the whole 24 hours.
When added to beta-blocker therapy, nicardipine further lowers both systolic and diastolic blood pressure.
-
INDICATIONS AND USAGE
I. Stable Angina
Nicardipine hydrochloride capsules are indicated for the management of patients with chronic stable angina (effort-associated angina). They may be used alone or in combination with beta-blockers.
II. Hypertension
Nicardipine hydrochloride capsules are indicated for the treatment of hypertension. They may be used alone or in combination with other antihypertensive drugs. In administering nicardipine hydrochloride it is important to be aware of the relatively large peak to trough differences in blood pressure effect. (See DOSAGE AND ADMINISTRATION.)
-
CONTRAINDICATIONS
Nicardipine hydrochloride is contraindicated in patients with hypersensitivity to the drug.
Because part of the effect of nicardipine is secondary to reduced afterload, the drug is also contraindicated in patients with advanced aortic stenosis. Reduction of diastolic pressure in these patients may worsen rather than improve myocardial oxygen balance.
-
WARNINGS
Increased Angina
About 7% of patients in short term placebo-controlled angina trials have developed increased frequency, duration or severity of angina on starting nicardipine or at the time of dosage increases, compared with 4% of patients on placebo. Comparisons with beta-blockers also show a greater frequency of increased angina, 4% vs. 1%. The mechanism of this effect has not been established. (See ADVERSE REACTIONS.)
Use in Patients with Congestive Heart Failure
Although preliminary hemodynamic studies in patients with congestive heart failure have shown that nicardipine reduced afterload without impairing myocardial contractility, it has a negative inotropic effect in vitro and in some patients. Caution should be exercised when using the drug in congestive heart failure patients, particularly in combination with a beta-blocker.
-
PRECAUTIONS
General
Blood Pressure
Because nicardipine decreases peripheral resistance, careful monitoring of blood pressure during the initial administration and titration of nicardipine is suggested. Nicardipine, like other calcium channel blockers, may occasionally produce symptomatic hypotension. Caution is advised to avoid systemic hypotension when administering the drug to patients who have sustained an acute cerebral infarction or hemorrhage. Because of prominent effects at the time of peak blood levels, initial titration should be performed with measurements of blood pressure at peak effect (1 to 2 hours after dosing) and just before the next dose.
Use in patients with impaired hepatic function
Since the liver is the major site of biotransformation and since nicardipine is subject to first pass metabolism, the drug should be used with caution in patients having impaired liver function or reduced hepatic blood flow. Patients with severe liver disease developed elevated blood levels (4-fold increase in AUC) and prolonged half-life (19 hours) of nicardipine. (See DOSAGE AND ADMINISTRATION.)
Use in patients with impaired renal function
When nicardipine hydrochloride 20 mg or 30 mg TID was given to hypertensive patients with mild renal impairment, mean plasma concentrations, AUC, and Cmax were approximately 2-fold higher in renally impaired patients than in healthy controls. Doses in these patients must be adjusted. (See CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION.)
Drug Interactions
Beta-Blockers
In controlled clinical studies, adrenergic beta-receptor blockers have been frequently administered concomitantly with nicardipine. The combination is well tolerated.
Cimetidine
Cimetidine increases nicardipine plasma levels. Patients receiving the two drugs concomitantly should be carefully monitored.
Digoxin
Some calcium blockers may increase the concentration of digitalis preparations in the blood. Nicardipine usually does not alter the plasma levels of digoxin, however, serum digoxin levels should be evaluated after concomitant therapy with nicardipine is initiated.
Aluminum and Magnesium Hydroxides
Coadministration of an antacid containing 600 mg aluminum hydroxide and 300 mg magnesium hydroxide had no effect on nicardipine absorption.
Fentanyl Anesthesia
Severe hypotension has been reported during fentanyl anesthesia with concomitant use of a beta-blocker and a calcium channel blocker. Even though such interactions were not seen during clinical studies with nicardipine, an increased volume of circulating fluids might be required if such an interaction were to occur.
Cyclosporine
Concomitant administration of nicardipine and cyclosporine results in elevated plasma cyclosporine levels. Plasma concentrations of cyclosporine should therefore be closely monitored, and its dosage reduced accordingly, in patients treated with nicardipine.
When therapeutic concentrations of furosemide, propranolol, dipyridamole, warfarin, quinidine, or naproxen were added to human plasma (in vitro), the plasma protein binding of nicardipine was not altered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Rats treated with nicardipine in the diet (at concentrations calculated to provide daily dosage levels of 5, 15 or 45 mg/kg/day) for 2 years showed a dose dependent increase in thyroid hyperplasia and neoplasia (follicular adenoma/carcinoma). One and 3 month studies in the rat have suggested that these results are linked to a nicardipine-induced reduction in plasma thyroxine (T4) levels with a consequent increase in plasma levels of thyroid stimulating hormone (TSH). Chronic elevation of TSH is known to cause hyperstimulation of the thyroid. In rats on an iodine deficient diet, nicardipine administration for one month was associated with thyroid hyperplasia that was prevented by T4 supplementation. Mice treated with nicardipine in the diet (at concentrations calculated to provide daily dosage levels of up to 100 mg/kg/day) for up to 18 months showed no evidence of neoplasia of any tissue and no evidence of thyroid changes. There was no evidence of thyroid pathology in dogs treated with up to 25 mg nicardipine/kg/day for one year and no evidence of effects of nicardipine on thyroid function (plasma T4 and TSH) in man.
There was no evidence of a mutagenic potential of nicardipine in a battery of genotoxicity tests conducted on microbial indicator organisms, in micronucleus tests in mice and hamsters, or in a sister chromatid exchange study in hamsters.
No impairment of fertility was seen in male or female rats administered nicardipine at oral doses as high as 100 mg/kg/day (50 times the 40 mg TID maximum recommended antianginal or antihypertensive dose in man, assuming a patient weight of 60 kg).
Pregnancy
Pregnancy Category C
Nicardipine was embryocidal when administered orally to pregnant Japanese White rabbits, during organogenesis, at 150 mg/kg/day (a dose associated with marked body weight gain suppression in the treated doe) but not at 50 mg/kg/day (25 times the maximum recommended antianginal or antihypertensive dose in man). No adverse effects on the fetus were observed when New Zealand albino rabbits were treated, during organogenesis, with up to 100 mg nicardipine/kg/day (a dose associated with significant mortality in the treated doe). In pregnant rats administered nicardipine orally at up to 100 mg/kg/day (50 times the maximum recommended human dose) there was no evidence of embryolethality or teratogenicity. However, dystocia, reduced birth weights, reduced neonatal survival and reduced neonatal weight gain were noted. There are no adequate and well controlled studies in pregnant women. Nicardipine should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Studies in rats have shown significant concentrations of nicardipine in maternal milk following oral administration. For this reason it is recommended that women who wish to breast-feed should not take this drug.
Geriatric Use
Pharmacokinetic parameters did not differ between elderly hypertensive patients (≥ 65 years) and healthy controls after one week of nicardipine treatment at 20 mg TID. Plasma nicardipine concentrations in elderly hypertensive patients were similar to plasma concentrations in healthy young adult subjects when nicardipine hydrochloride was administered at doses of 10 mg, 20 mg and 30 mg TID, suggesting that the pharmacokinetics of nicardipine are similar in young and elderly hypertensive patients.
Clinical studies of nicardipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
-
ADVERSE REACTIONS
In multiple-dose U.S. and foreign controlled short-term (up to 3 months) studies 1,910 patients received nicardipine alone or in combination with other drugs. In these studies adverse events were reported spontaneously; adverse experiences were generally not serious but occasionally required dosage adjustment and about 10% of patients left the studies prematurely because of them. Peak responses were not observed to be associated with adverse effects during clinical trials, but physicians should be aware that adverse effects associated with decreases in blood pressure (tachycardia, hypotension, etc.) could occur around the time of the peak effect. Most adverse effects were expected consequences of the vasodilator effects of nicardipine.
Angina
The incidence rates of adverse effects in anginal patients were derived from multicenter, controlled clinical trials. Following are the rates of adverse effects for nicardipine (n = 520) and placebo (n = 310), respectively, that occurred in 0.4% of patients or more. These represent events considered probably drug-related by the investigator (except for certain cardiovascular events which were recorded in a different category). Where the frequency of adverse effects for nicardipine and placebo is similar, causal relationship is uncertain. The only dose related effects were pedal edema and increased angina.
Percent of Patients with Adverse Effects in Controlled Studies (Incidence of discontinuations shown in parentheses) NICARDIPINE PLACEBO Adverse Experience (n = 520) (n = 310) Pedal Edema
7.1 (0)
0.3 (0)
Dizziness
6.9 (1.2)
0.6 (0)
Headache
6.4 (0.6)
2.6 (0)
Asthenia
5.8 (0.4)
2.6 (0)
Flushing
5.6 (0.4)
1 (0)
Increased Angina
5.6 (3.5)
4.2 (1.9)
Palpitations
3.3 (0.4)
0 (0)
Nausea
1.9 (0)
0.3 (0)
Dyspepsia
1.5 (0.6)
0.6 (0.3)
Dry Mouth
1.4 (0)
0.3 (0)
Somnolence
1.4 (0)
1 (0)
Rash
1.2 (0.2)
0.3 (0)
Tachycardia
1.2 (0.2)
0.6 (0)
Myalgia
1 (0)
0 (0)
Other edema
1 (0)
0 (0)
Paresthesia
1 (0.2)
0.3 (0)
Sustained Tachycardia
0.8 (0.6)
0 (0)
Syncope
0.8 (0.2)
0 (0)
Constipation
0.6 (0.2)
0.6 (0)
Dyspnea
0.6 (0)
0 (0)
Abnormal ECG
0.6 (0.6)
0 (0)
Malaise
0.6 (0)
0 (0)
Nervousness
0.6 (0)
0.3 (0)
Tremor
0.6 (0)
0 (0)
In addition, adverse events were observed which are not readily distinguishable from the natural history of the atherosclerotic vascular disease in these patients. Adverse events in this category each occurred in <0.4% of patients receiving nicardipine and included myocardial infarction, atrial fibrillation, exertional hypotension, pericarditis, heart block, cerebral ischemia and ventricular tachycardia. It is possible that some of these events were drug-related.
Hypertension
The incidence rates of adverse effects in hypertensive patients were derived from multicenter, controlled clinical trials. Following are the rates of adverse effects for nicardipine (n = 1390) and placebo (n = 211), respectively, that occurred in 0.4% of patients or more. These represent events considered probably drug-related by the investigator. Where the frequency of adverse effects for nicardipine and placebo is similar, causal relationship is uncertain. The only dose-related effect was pedal edema.
Percent of Patients with Adverse Effects in Controlled Studies (Incidence of discontinuations shown in parentheses) NICARDIPINE PLACEBO Adverse Experience (n=1,390) (n=211) Flushing
9.7 (2.1)
2.8 (0)
Headache
8.2 (2.6)
4.7 (0)
Pedal Edema
8 (1.8)
0.9 (0)
Asthenia
4.2 (1.7)
0.5 (0)
Palpitations
4.1 (1)
0 (0)
Dizziness
4 (1.8)
0 (0)
Tachycardia
3.4 (1.2)
0.5 (0)
Nausea
2.2 (0.9)
0.9 (0)
Somnolence
1.1 (0.1)
0 (0)
Dyspepsia
0.8 (0.3)
0.5 (0)
Insomnia
0.6 (0.1)
0 (0)
Malaise
0.6 (0.1)
0 (0)
Other edema
0.6 (0.3)
1.4 (0)
Abnormal dreams
0.4 (0)
0 (0)
Dry mouth
0.4 (0.1)
0 (0)
Nocturia
0.4 (0)
0 (0)
Rash
0.4 (0.4)
0 (0)
Vomiting
0.4 (0.4)
0 (0)
Rare Events
The following rare adverse events have been reported in clinical trials or the literature:
Body as a Whole: infection, allergic reaction
Cardiovascular: hypotension, postural hypotension, atypical chest pain, peripheral vascular disorder, ventricular extrasystoles, ventricular tachycardia
Digestive: sore throat, abnormal liver chemistries
Musculoskeletal: arthralgia
Nervous: hot flashes, vertigo, hyperkinesia, impotence, depression, confusion, anxiety
Respiratory: rhinitis, sinusitis
Special Senses: tinnitus, abnormal vision, blurred vision
Urogenital: increased urinary frequency
-
OVERDOSAGE
Overdosage with a 600 mg single dose (15 to 30 times normal clinical dose) has been reported. Marked hypotension (blood pressure unobtainable) and bradycardia (heart rate 20 bpm in normal sinus rhythm) occurred, along with drowsiness, confusion and slurred speech. Supportive treatment with a vasopressor resulted in gradual improvement with normal vital signs approximately 9 hours post treatment.
Based on results obtained in laboratory animals, overdosage may cause systemic hypotension, bradycardia (following initial tachycardia) and progressive atrioventricular conduction block. Reversible hepatic function abnormalities and sporadic focal hepatic necrosis were noted in some animal species receiving very large doses of nicardipine.
For treatment of overdose standard measures (for example, evacuation of gastric contents, elevation of extremities, attention to circulating fluid volume and urine output) including monitoring of cardiac and respiratory functions should be implemented. The patient should be positioned so as to avoid cerebral anoxia. Frequent blood pressure determinations are essential. Vasopressors are clinically indicated for patients exhibiting profound hypotension. Intravenous calcium gluconate may help reverse the effects of calcium entry blockade.
-
DOSAGE AND ADMINISTRATION
Angina
The dose should be individually titrated for each patient beginning with 20 mg 3 times daily. Doses in the range of 20 mg to 40 mg 3 times a day have been shown to be effective. At least 3 days should be allowed before increasing the nicardipine hydrochloride dose to ensure achievement of steady-state plasma drug concentrations.
Concomitant Use With Other Antianginal Agents
- 1. Sublingual NTG may be taken as required to abort acute anginal attacks during nicardipine therapy.
- 2. Prophylactic Nitrate Therapy–Nicardipine may be safely coadministered with short- and long-acting nitrates.
- 3. Beta-blockers–(See PRECAUTIONS, Drug Interactions.)
Hypertension
The dose of nicardipine hydrochloride should be individually adjusted according to the blood pressure response beginning with 20 mg 3 times daily. The effective doses in clinical trials have ranged from 20 mg to 40 mg 3 times daily. The maximum blood pressure lowering effect occurs approximately 1 to 2 hours after dosing. To assess the adequacy of blood pressure response, the blood pressure should be measured at trough (8 hours after dosing). Because of the prominent peak effects of nicardipine, blood pressure should be measured 1 to 2 hours after dosing, particularly during initiation of therapy. (See PRECAUTIONS, Blood Pressure; INDICATIONS AND USAGE; CLINICAL PHARMACOLOGY, Effects in Hypertension.) At least 3 days should be allowed before increasing the nicardipine dose to ensure achievement of steady-state plasma drug concentrations.
Special Patient Populations
Renal Insufficiency
Although there is no evidence that nicardipine impairs renal function, careful dose titration beginning with 20 mg TID is advised. (See PRECAUTIONS.)
Hepatic Insufficiency
Nicardipine should be administered cautiously in patients with severely impaired hepatic function. A suggested starting dose of 20 mg twice a day is advised with individual titration based on clinical findings maintaining the twice a day schedule. (See PRECAUTIONS.)
Congestive Heart Failure
Caution is advised when titrating nicardipine dosage in patients with congestive heart failure. (See WARNINGS.)
- HOW SUPPLIED
- NICARDIPINE HYDROCHLORIDE CAPSULE
-
INGREDIENTS AND APPEARANCE
NICARDIPINE HYDROCHLORIDE
nicardipine hydrochloride capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 68151-0092(NDC:0378-1020) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength NICARDIPINE HYDROCHLORIDE (UNII: K5BC5011K3) (NICARDIPINE - UNII:CZ5312222S) NICARDIPINE HYDROCHLORIDE 20 mg Inactive Ingredients Ingredient Name Strength SILICON DIOXIDE (UNII: ETJ7Z6XBU4) GELATIN (UNII: 2G86QN327L) MAGNESIUM STEARATE (UNII: 70097M6I30) STARCH, CORN (UNII: O8232NY3SJ) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERROSOFERRIC OXIDE (UNII: XM0M87F357) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) Product Characteristics Color BLUE (medium blue-green opaque) , WHITE (ivory opaque) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code MYLAN;1020 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68151-0092-7 1 in 1 PACKAGE; Type 0: Not a Combination Product 07/19/1996 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074642 07/19/1996 Labeler - Carilion Materials Management (079239644) Establishment Name Address ID/FEI Business Operations Carilion Materials Management 079239644 REPACK(68151-0092)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.