DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE tablet
Diphenoxylate hydrochloride and atropine sulfate by
Drug Labeling and Warnings
Diphenoxylate hydrochloride and atropine sulfate by is a Prescription medication manufactured, distributed, or labeled by Chartwell RX, LLC, ECI Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Each Diphenoxylate Hydrochloride and Atropine Sulfate Tablet, USP contains:
2.5 mg of diphenoxylate hydrochloride USP (equivalent to 2.3 mg of diphenoxylate) and 0.025 mg of atropine sulfate USP (equivalent to 0.01 mg of atropine).
Diphenoxylate hydrochloride, an antidiarrheal, is ethyl 1-(3-cyano-3,3-diphenylpropyl)-4-phenylisonipecotate monohydrochloride and has the following structural formula:
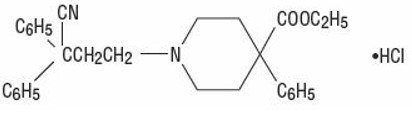
Atropine sulfate, an anticholinergic, is endo-(±)-α-(hydroxymethyl) benzeneacetic acid 8-methyl-8-azabicyclo[3.2.1] oct-3-yl ester sulfate (2:1) (salt) monohydrate and has the following structural formula:
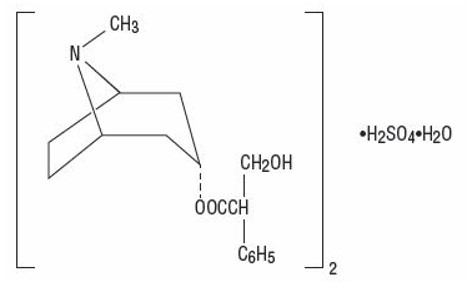
A subtherapeutic amount of atropine sulfate is present to discourage deliberate overdosage.
Inactive ingredients of diphenoxylate hydrochloride and atropine sulfate tablets include corn starch, hypromellose, lactose monohydrate, magnesium stearate, polyethylene glycol, sucralose and talc.
-
CLINICAL PHARMACOLOGY
Diphenoxylate is rapidly and extensively metabolized in man by ester hydrolysis to diphenoxylic acid (difenoxine), which is biologically active and the major metabolite in the blood. After a 5-mg oral dose of carbon-14 labeled diphenoxylate hydrochloride in ethanolic solution was given to three healthy volunteers, an average of 14% of the drug plus its metabolites was excreted in the urine and 49% in the feces over a four-day period. Urinary excretion of the unmetabolized drug constituted less than 1% of the dose, and diphenoxylic acid plus its glucuronide conjugate constituted about 6% of the dose. In a 16-subject crossover bioavailability study, a linear relationship in the dose range of 2.5 to 10 mg was found between the dose of diphenoxylate hydrochloride (given as diphenoxylate hydrochloride and atropine sulfate liquid) and the peak plasma concentration, the area under the plasma concentration-time curve, and the amount of diphenoxylic acid excreted in the urine. In the same study the bioavailability of the tablet compared with an equal dose of the liquid was approximately 90%. The average peak plasma concentration of diphenoxylic acid following ingestion of four 2.5-mg tablets was 163 ng/ml at about 2 hours, and the elimination half-life of diphenoxylic acid was approximately 12 to 14 hours.
In dogs, diphenoxylate hydrochloride has a direct effect on circular smooth muscle of the bowel that conceivably results in segmentation and prolongation of gastrointestinal transit time. The clinical antidiarrheal action of diphenoxylate hydrochloride may thus be a consequence of enhanced segmentation that allows increased contact of the intraluminal contents with the intestinal mucosa.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Diphenoxylate hydrochloride and atropine sulfate tablets are contraindicated in:
- Pediatric patients less than 6 years of age due to the risks of respiratory and central nervous system (CNS) depression (see WARNINGS).
- Patients with diarrhea associated with pseudomembranous enterocolitis ( Clostridium difficile) or other enterotoxin-producing bacteria due to the risk of gastrointestinal (GI) complications, including sepsis (see WARNINGS).
- Patients with known hypersensitivity to diphenoxylate or atropine.
- Patients with obstructive jaundice.
-
WARNINGS
Respiratory and/or CNS Depression in Pediatric Patients Less Than 6 Years of Age
Cases of severe respiratory depression and coma, leading to permanent brain damage or death have been reported in patients less than 6 years of age who received diphenoxylate hydrochloride and atropine sulfate tablets . Diphenoxylate hydrochloride and atropine sulfate tablets are contraindicated in patients less than 6 years of age due to these risks (see CONTRAINDICATIONS).
Anticholinergic and Opioid-Toxicities
Toxicities associated with the atropine and diphenoxylate components of diphenoxylate hydrochloride and atropine sulfate tablets have been reported. The initial presenting symptoms may be delayed by up to 30 hours due to prolonged gastric emptying time induced by diphenoxylate hydrochloride. Clinical presentations vary in terms of which toxicity (anticholinergic vs. opioid) will present first or predominate; non-specific findings have been reported and include symptoms such as drowsiness (see OVERDOSAGE).
Dehydration and Electrolyte Imbalance
The use of diphenoxylate hydrochloride and atropine sulfate tablets should be accompanied by appropriate fluid and electrolyte therapy, when indicated. If severe dehydration or electrolyte imbalance is present, diphenoxylate hydrochloride and atropine sulfate tablets should be withheld until appropriate corrective therapy has been initiated. Drug-induced inhibition of peristalsis may result in fluid retention in the intestine, which may further aggravate dehydration and electrolyte imbalance.
Gastrointestinal Complications in Patients with Infectious Diarrhea
Diphenoxylate hydrochloride and atropine sulfate tablets are contraindicated in patients with diarrhea associated with organisms that penetrate the GI mucosa (toxigenic E. coli, Salmonella, Shigella), and pseudomembranous enterocolitis ( Clostridium difficile) associated with broad-spectrum antibiotics (see CONTRAINDICATIONS). Antiperistaltic agents, including diphenoxylate hydrochloride and atropine sulfate tablets, slow gastrointestinal motility and may enhance bacterial overgrowth and the release of bacterial exotoxins. Diphenoxylate hydrochloride and atropine sulfate tablets have been reported to result in serious GI complications in patients with infectious diarrhea, including sepsis, prolonged and/or worsened diarrhea. Prolonged fever and the delay in the resolution of stool pathogens were reported in study of Shigellosis in adults who used diphenoxylate hydrochloride and atropine sulfate tablets vs. placebo.
Toxic Megacolon in Patients with Acute Ulcerative Colitis
In some patients with acute ulcerative colitis, agents that inhibit intestinal motility or prolong intestinal transit time have been reported to induce toxic megacolon. Consequently, patients with acute ulcerative colitis should be carefully observed and diphenoxylate hydrochloride and atropine sulfate tablets therapy should be discontinued promptly if abdominal distention occurs or if other untoward symptoms develop.
Interaction with Meperidine Hydrochloride
Since the chemical structure of diphenoxylate hydrochloride is similar to that of meperidine hydrochloride, the concurrent use of diphenoxylate hydrochloride and atropine sulfate tablets with monoamine oxidase (MAO) inhibitors may, in theory, precipitate hypertensive crisis.
Hepatorenal Disease
Diphenoxylate hydrochloride and atropine sulfate tablets should be used with extreme caution in patients with advanced hepatorenal disease and in all patients with abnormal liver function since hepatic coma may be precipitated.
Interaction with CNS Depressants
Diphenoxylate hydrochloride and atropine sulfate tablets may potentiate the action of other drugs that cause dizziness or drowsiness, including barbiturates, benzodiazepines and other sedatives/hypnotics, anxiolytics, and tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, and alcohol. Therefore, the patient should be closely observed when any of these are used concomitantly.
-
PRECAUTIONS
Atropinism
Since a subtherapeutic dose of atropine has been added to diphenoxylate hydrochloride and atropine sulfate tablets, consideration should be given to the development of adverse reactions associated with atropine (see WARNINGS). Diphenoxylate hydrochloride and atropine sulfate tablets have caused atropinism (hyperthermia, tachycardia, urinary retention, flushing, dryness of the skin and mucous membranes) particularly in pediatric patients with Down's syndrome. Diphenoxylate hydrochloride and atropine sulfate tablets are not indicated for use in pediatric patients (see CONTRAINDICATIONS and WARNINGS) . Monitor patients for signs of atropinism.
Information for patients
Advise patients:
- Accidental ingestion of diphenoxylate hydrochloride and atropine sulfate tablets in children, especially in those less than 6 years of age, may result in severe respiratory depression or coma. Instruct patients to take steps to store diphenoxylate hydrochloride and atropine sulfate tablets securely and out of reach of children, and to dispose of unused diphenoxylate hydrochloride and atropine sulfate tablets (see WARNINGS).
- To take diphenoxylate hydrochloride and atropine sulfate tablets at the prescribed dosage. Use of a higher than prescribed dosage may include opioid and/or anticholinergic effects (see OVERDOSAGE). Report to a healthcare facility if they develop anticholinergic symptoms such as hyperthermia, flushing, tachycardia, tachypnea, hypotonia, lethargy, hallucinations, febrile convulsion, dry mouth, mydriasis or opioid symptoms such as progressive CNS and respiratory depression, miosis, seizures, or paralytic ileus.
- Diphenoxylate hydrochloride and atropine sulfate tablets may produce drowsiness or dizziness. Concomitant use of alcohol or other drugs that also cause CNS depression (e.g., barbiturates, benzodiazepines, opioids, buspirone, antihistamines, and muscle relaxants) may increase this effect. Inform patients not to operate motor vehicles or other dangerous machinery until they are reasonably certain that diphenoxylate hydrochloride and atropine sulfate tablets does not affect them adversely.
- To use fluid and electrolyte therapy, if prescribed along with diphenoxylate hydrochloride and atropine sulfate tablets, as instructed by their healthcare provider.
- Clinical improvement of diarrhea is usually observed within 48 hours. If clinical improvement is not seen within 10 days, discontinue diphenoxylate hydrochloride and atropine sulfate tablets and contact their healthcare provider.
Drug interactions
Alcohol
Alcohol may increase the CNS depressant effects of diphenoxylate hydrochloride and atropine sulfate tablets and may cause drowsiness (see WARNINGS). Avoid concomitant use of diphenoxylate hydrochloride and atropine sulfate tablets with alcohol.
Other Drugs that Cause CNS Depression
The concurrent use of diphenoxylate hydrochloride and atropine sulfate tablets with other drugs that cause CNS depression (e.g., barbiturates, benzodiazepines, opioids, buspirone, antihistamines, muscle relaxants), may potentiate the effects of diphenoxylate hydrochloride and atropine sulfate tablets (see WARNINGS). Either diphenoxylate hydrochloride and atropine sulfate tablets or the other interacting drug should be chosen, depending on the importance of the drug to the patient. If CNS-acting drugs cannot be avoided, monitor patients for CNS adverse reactions.
MAO Inhibitors
Diphenoxylate may interact with monoamine oxidase inhibitors (MAOIs) and precipitate a hypertensive crisis . Avoid use of diphenoxylate hydrochloride and atropine sulfate tablets in patients who take MAOIs and monitor for signs and symptoms of hypertensive crisis (headache, hyperthermia, hypertension).
Carcinogenesis, mutagenesis, impairment of fertility
No long-term study in animals has been performed to evaluate carcinogenic potential. Diphenoxylate hydrochloride was administered to male and female rats in their diets to provide dose levels of 4 and 20 mg/kg/day throughout a three-litter reproduction study. At 50 times the human dose (20 mg/kg/day), female weight gain was reduced and there was a marked effect on fertility as only 4 of 27 females became pregnant in three test breedings. The relevance of this finding to usage of diphenoxylate hydrochloride and atropine sulfate tablets in humans is unknown.
Pregnancy
Diphenoxylate hydrochloride has been shown to have an effect on fertility in rats when given in doses 50 times the human dose (see above discussion). Other findings in this study include a decrease in maternal weight gain of 30% at 20 mg/kg/day and of 10% at 4 mg/kg/day. At 10 times the human dose (4 mg/kg/day), average litter size was slightly reduced.
Teratology studies were conducted in rats, rabbits, and mice with diphenoxylate hydrochloride at oral doses of 0.4 to 20 mg/kg/day. Due to experimental design and small numbers of litters, embryotoxic, fetotoxic, or teratogenic effects cannot be adequately assessed. However, examination of the available fetuses did not reveal any indication of teratogenicity.
There are no adequate and well-controlled studies in pregnant women. Diphenoxylate hydrochloride and atropine sulfate tablets should be used during pregnancy only if the anticipated benefit justifies the potential risk to the fetus.
Nursing mothers
Caution should be exercised when diphenoxylate hydrochloride and atropine sulfate tablets are administered to a nursing woman, since the physicochemical characteristics of the major metabolite, diphenoxylic acid, are such that it may be excreted in breast milk and since it is known that atropine is excreted in breast milk.
Pediatric use
The safety and effectiveness of diphenoxylate hydrochloride and atropine sulfate tablets have been established in pediatric patients 13 years of age and older as adjunctive therapy in the management of diarrhea. The safety and effectiveness of diphenoxylate hydrochloride and atropine sulfate tablets have not been established in pediatric patients less than 13 years of age.
Diphenoxylate hydrochloride and atropine sulfate tablets are contraindicated in pediatric patients less than 6 years of age due to the risks of severe respiratory depression and coma, possibly resulting in permanent brain damage or death (see CONTRAINDICATIONS).
Diphenoxylate hydrochloride and atropine sulfate tablets have caused atropinism, particularly in pediatric patients with Down's syndrome (see PRECAUTIONS).
In case of accidental ingestion of diphenoxylate hydrochloride and atropine sulfate tablets by pediatric patients, see OVERDOSAGE for recommended treatment.
-
ADVERSE REACTIONS
The following serious adverse reactions are described elsewhere in labeling:
- Respiratory and/or CNS depression (see WARNINGS)
- Anticholinergic and opioid-toxicities, including atroponism (see WARNINGS and PRECAUTIONS)
- Dehydration and electrolyte imbalance (see WARNINGS)
- GI Complications in patients with infectious diarrhea (see WARNINGS)
- Toxic megacolon in patients with acute ulcerative colitis (see WARNINGS)
At therapeutic doses of diphenoxylate hydrochloride and atropine sulfate tablets, the following other adverse reactions have been reported; they are listed in decreasing order of severity, but not of frequency:
Nervous system: numbness of extremities, euphoria, depression, malaise/lethargy, confusion, sedation/drowsiness, dizziness, restlessness, headache, hallucination
Allergic: anaphylaxis, angioneurotic edema, urticaria, swelling of the gums, pruritus.
Gastrointestinal system: megacolon, paralytic ileus, pancreatitis, vomiting, nausea, anorexia, abdominal discomfort
The following adverse reactions related to atropine sulfate are listed in decreasing order of severity, but not of frequency: hyperthermia, tachycardia, urinary retention, flushing, dryness of the skin and mucous membranes.
-
DRUG ABUSE AND DEPENDENCE
Controlled substance
Diphenoxylate hydrochloride and atropine sulfate tablets are classified as a Schedule V controlled substance by federal regulation. Diphenoxylate hydrochloride is chemically related to the narcotic analgesic meperidine.
Drug abuse and dependence
In doses used for the treatment of diarrhea, whether acute or chronic, diphenoxylate has not produced addiction.
Diphenoxylate hydrochloride is devoid of morphine-like subjective effects at therapeutic doses. At high doses it exhibits codeine-like subjective effects. The dose which produces antidiarrheal action is widely separated from the dose which causes central nervous system effects. The insolubility of diphenoxylate hydrochloride in commonly available aqueous media precludes intravenous self-administration. A dose of 100 to 300 mg/day, which is equivalent to 40 to 120 tablets, administered to humans for 40 to 70 days, produced opiate withdrawal symptoms. Since addiction to diphenoxylate hydrochloride is possible at high doses, the recommended dosage should not be exceeded.
-
OVERDOSAGE
Diagnosis
Overdosage can be life-threatening. Symptoms of overdosage may include opioid and/or anticholinergic effects including respiratory depression, coma, delirium, lethargy, dryness of the skin and mucous membranes, mydriasis or miosis, flushing, hyperthermia, tachycardia, hypotonia, tachypnea, toxic encephalopathy, seizures and incoherent speech.
Respiratory depression has been reported up to 30 hours after ingestion and may recur despite an initial response to narcotic antagonists.
Treat all possible diphenoxylate hydrochloride and atropine sulfate tablets overdosages as serious and maintain medical observation/hospitalization until patients become asymptomatic without naloxone use.
Treatment
A pure narcotic antagonist (e.g., naloxone) should be used in the treatment of respiratory depression caused by diphenoxylate hydrochloride and atropine sulfate tablets. Refer to the prescribing information for naloxone. Consider diphenoxylate hydrochloride and atropine sulfate tablets toxicity even in settings of negative toxicology tests.
Following initial improvement of respiratory function, repeated doses of naloxone hydrochloride may be required to counteract recurrent respiratory depression.
If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
-
DOSAGE AND ADMINISTRATION
Management of Diarrhea in Patients 13 Years of Age and Older
Diphenoxylate hydrochloride and atropine sulfate tablets are recommended as adjunctive therapy for the management of diarrhea in patients 13 years of age and older. Consider the nutritional status and degree of dehydration in patients prior to initiating therapy with diphenoxylate hydrochloride and atropine sulfate tablets. The use of diphenoxylate hydrochloride and atropine sulfate tablets should be accompanied by appropriate fluid and electrolyte therapy, when indicated. If severe dehydration or electrolyte imbalance is present, do not administer diphenoxylate hydrochloride and atropine sulfate tablets until appropriate corrective therapy has been indicated (see WARNINGS).
Initial and Maximum Recommended Dosage in Patients 13 Years of Age and Older
The initial adult dosage is 2 diphenoxylate hydrochloride and atropine sulfate tablets four times daily (maximum total daily dose of 20 mg per day of diphenoxylate hydrochloride). Most patients will require this dosage until initial control of diarrhea has been achieved. Clinical improvement of acute diarrhea is usually observed within 48 hours.
Dosage after Initial Control of Diarrhea
After initial control has been achieved, the diphenoxylate hydrochloride and atropine sulfate tablets dosage may be reduced to meet individual requirements. Control may often be maintained with as little as two diphenoxylate hydrochloride and atropine sulfate tablets daily.
Duration of Treatment
If clinical improvement of chronic diarrhea after treatment with the maximum recommended daily dosage is not observed within 10 days, discontinue diphenoxylate hydrochloride and atropine sulfate tablets as symptoms are unlikely to be controlled by further administration.
-
HOW SUPPLIED
Diphenoxylate Hydrochloride and Atropine Sulfate Tablets USP are available as white round tablets, debossed "ECI" on one side and "618" on the other side. Each tablet contains 2.5 mg of diphenoxylate hydrochloride and 0.025 mg of atropine sulfate, supplied as:
NDC NumberSize
62135-618-12 bottle of 120
Store at 20º to 25ºC (68º to 77ºF); excursions permitted to 15°- 30°C (59°-86°F) [See USP Controlled Room Temperature].
Rx only
Distributed By:
Chartwell RX, LLC
Congers, NY, 10920
Revised 05/2022L70904
-
PACKAGE LABEL.PRINICIPAL DISPLAY PANEL
Diphenoxylate Hydrochloride and Atropine Sulfate Tablet, USP (2.5/0.025 mg) - NDC: 62135-618-12 - 120's Bottle Label

-
INGREDIENTS AND APPEARANCE
DIPHENOXYLATE HYDROCHLORIDE AND ATROPINE SULFATE
diphenoxylate hydrochloride and atropine sulfate tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 62135-618 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENOXYLATE HYDROCHLORIDE (UNII: W24OD7YW48) (DIPHENOXYLATE - UNII:73312P173G) DIPHENOXYLATE HYDROCHLORIDE 2.5 mg ATROPINE SULFATE (UNII: 03J5ZE7KA5) (ATROPINE - UNII:7C0697DR9I) ATROPINE SULFATE 0.025 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SUCRALOSE (UNII: 96K6UQ3ZD4) TALC (UNII: 7SEV7J4R1U) Product Characteristics Color white Score no score Shape ROUND Size 6mm Flavor Imprint Code ECI;618 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 62135-618-12 120 in 1 BOTTLE; Type 0: Not a Combination Product 06/08/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207128 10/21/2020 Labeler - Chartwell RX, LLC (079394054)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.