SAL INGLESA EPSOM SALT- magnesium sulfate heptahydrate crystal
Sal Inglesa by
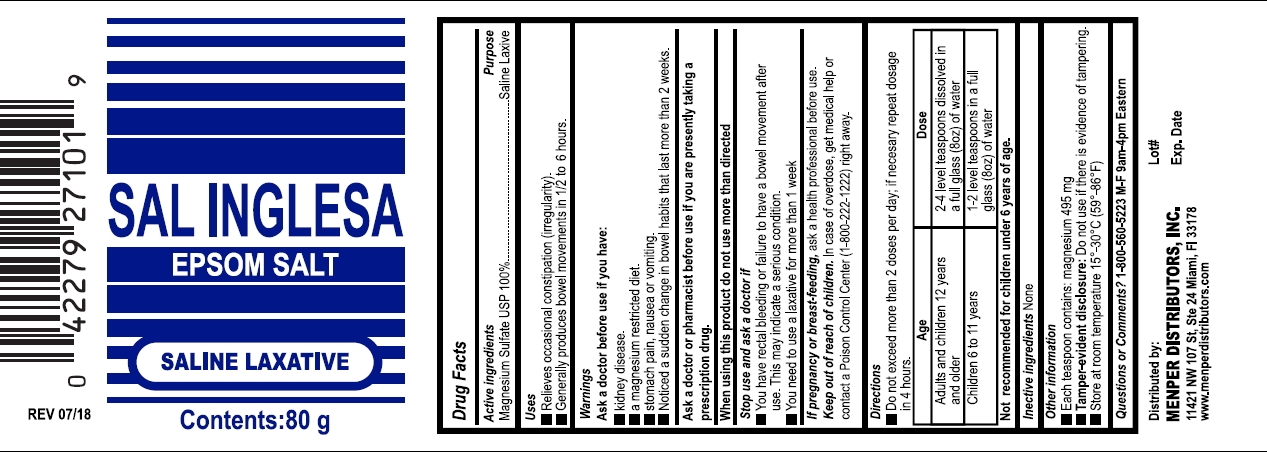
Drug Labeling and Warnings
Sal Inglesa by is a Otc medication manufactured, distributed, or labeled by Menper Distributors, Inc, DRLZ Enterprises LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredient & Purposes
- Uses
-
Warnings
Ask a doctor before use if you have
- kidney disease
- a magnesium-restricted diet
- stomach pain, nausea, or vomiting
- noticed a sudden change in bowel habits that last more than 2 weeks
-
Directions
- tbsp = tablespoon
- drink a full glass (8 ounces) of liquid with each dose. The dose may be taken as a single daily dose or in divided doses.
- do not exceed maximum daily dosage, unless directed by a doctor
adults and children 12 years of age and older
2-4 level teaspoons dissolved in a full glass (8oz) of water
children 6 to under 12 years of age 1-2 level teaspoons dissolved in a full glass (8oz) of water children under 6 years of age
Not recomended - Other information
- Inactive ingredient
- Questions?
- Prinicpal Display Panel
-
INGREDIENTS AND APPEARANCE
SAL INGLESA EPSOM SALT
magnesium sulfate heptahydrate crystalProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 53145-580 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MAGNESIUM SULFATE HEPTAHYDRATE (UNII: SK47B8698T) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM SULFATE HEPTAHYDRATE 1 g in 1 g Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 53145-580-01 80 g in 1 BOTTLE; Type 0: Not a Combination Product 07/01/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M007 07/01/2025 Labeler - Menper Distributors, Inc (101947166) Establishment Name Address ID/FEI Business Operations DRLZ Enterprises LLC 031125329 manufacture(53145-580)
Trademark Results [Sal Inglesa]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 SAL INGLESA 73717743 not registered Dead/Abandoned |
IBERO-AMERICANO CORPORATION 1988-03-21 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.