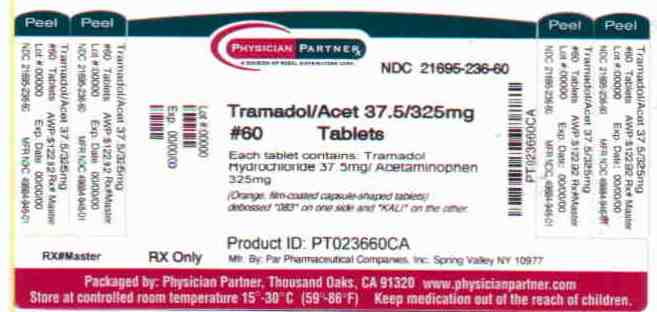
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN tablet
Tramadol Hydrochloride and Acetaminophen by
Drug Labeling and Warnings
Tramadol Hydrochloride and Acetaminophen by is a Prescription medication manufactured, distributed, or labeled by Rebel Distributors Corp. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Tramadol hydrochloride and acetaminophen tablets, 37.5 mg/325 mg, combines two analgesics, tramadol and acetaminophen.
The chemical name for tramadol hydrochloride is (±)cis-2-[(dimethylamino)methyl]-1-(3-methoxyphenyl) cyclohexanol hydrochloride. Its structural formula is:
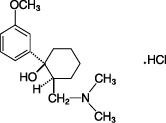
The molecular weight of tramadol hydrochloride is 299.84. Tramadol hydrochloride is a white, bitter, crystalline and odorless powder.
The chemical name for acetaminophen is N-acetyl-p-aminophenol. Its structural formula is:

The molecular weight of acetaminophen is 151.17. Acetaminophen is an analgesic and antipyretic agent which occurs as a white, odorless, crystalline powder, possessing a slightly bitter taste.
Tramadol hydrochloride and acetaminophen tablets contain 37.5 mg tramadol hydrochloride and 325 mg acetaminophen and are orange in color. Tramadol hydrochloride and acetaminophen tablets are intended for oral administration. Inactive ingredients in the tablet are crospovidone, magnesium stearate, microcrystalline cellulose, OPADRY® II Orange, povidone, pregelatinized starch, purified water, and stearic acid. OPADRY® II Orange contains: FD&C red #40; FD&C yellow #6; hypromellose 2910 3cP, 6cP, and 50cP; polydextrose; polyethylene glycol; titanium dioxide; triacetate; and triacetin.
-
CLINICAL PHARMACOLOGY
The following information is based on studies of tramadol alone or acetaminophen alone, except where otherwise noted:
Pharmacodynamics
Tramadol
Tramadol is a centrally acting synthetic opioid analgesic. Although its mode of action is not completely understood, from animal tests, at least two complementary mechanisms appear applicable: binding of parent and M1 metabolite to µ-opioid receptors and weak inhibition of reuptake of norepinephrine and serotonin.
Opioid activity is due to both low affinity binding of the parent compound and higher affinity binding of the O-demethylated metabolite M1 to µ-opioid receptors. In animal models, M1 is up to 6 times more potent than tramadol in producing analgesia and 200 times more potent in µ-opioid binding. Tramadol-induced analgesia is only partially antagonized by the opiate antagonist naloxone in several animal tests. The relative contribution of both tramadol and M1 to human analgesia is dependent upon the plasma concentrations of each compound (see CLINICAL PHARMAMCOLOGY, Pharmacokinetics).
Tramadol has been shown to inhibit reuptake of norepinephrine and serotonin in vitro, as have some other opioid analgesics. These mechanisms may contribute independently to the overall analgesic profile of tramadol. Apart from analgesia, tramadol administration may produce a constellation of symptoms (including dizziness, somnolence, nausea, constipation, sweating and pruritis) similar to that of other opioids.
Pharmacokinetics
Tramadol is administered as a racemate and both the [-] and [+] forms of both tramadol and M1 are detected in the circulation. The pharmacokinetics of plasma tramadol and acetaminophen following oral administration of one tramadol hydrochloride and acetaminophen tablet are shown in Table 1. Tramadol has a slower absorption and longer half-life when compared to acetaminophen.
Table 1: Summary of Mean (±SD) Pharmacokinetic Parameters of the (+) – and (-) Enantiomers of Tramadol and M1 and Acetaminophen Following A Single Oral Dose of One Tramadol Hydrochloride and Acetaminophen Combination Tablet in Volunteers a For acetaminophen Cmax was measured in mcg/mL.
Parameter[1] (+)-Tramadol (-)-Tramadol (+)-M1 (-)-M1 Acetaminophen Cmax (ng/mL) 64.3 (9.3) 55.5 (8.1) 10.9 (5.7) 12.8 (4.2) 4.2 (0.8) tmax(h) 1.8 (0.6) 1.8 (0.7) 2.1 (0.7) 2.2 (0.7) 0.9 (0.7) CL/F (mL/min) 588 (226) 736 (244) - - - - 365 (84) t½ (h) 5.1 (1.4) 4.7 (1.2) 7.8 (3.0) 6.2 (1.6) 2.5 (0.6) A single dose pharmacokinetic study of tramadol hydrochloride and acetaminophen tablets in volunteers showed no drug interactions between tramadol and acetaminophen. Upon multiple oral dosing to steady state, however, the bioavailability of tramadol and metabolite M1 was lower for the combination tablets compared to tramadol administered alone. The decrease In AUC was 14% for (+)-tramadol, 10.4% for (-)-tramadol, 11.9% for (+)-M1 and 24.2% for (-)-M1. The cause of this reduced bioavailability is not clear. Following single or multiple dose administration of tramadol hydrochloride and acetaminophen tablets, no significant change in acetaminophen pharmacokinetics was observed when compared to acetaminophen given alone.
Absorption:
The absolute bioavailability of tramadol from tramadol hydrochloride and acetaminophen tablets has not been determined. Tramadol hydrochloride has a mean absolute bioavailability of approximately 75% following administration of a single 100 mg oral dose of Tramadol HCl tablets. The mean peak plasma concentration of racemic tramadol and M1 after administration of two tramadol hydrochloride and acetaminophen tablets occurs at approximately two and three hours, respectively, post-dose.
Peak plasma concentrations of acetaminophen occur within one hour and are not affected by co-administration with tramadol. Oral absorption of acetaminophen following administration of tramadol hydrochloride and acetaminophen tablets occurs primarily in the small intestine.
Food Effects:
When tramadol hydrochloride and acetaminophen tablets were administered with food, the time to peak plasma concentration was delayed for approximately 35 minutes for tramadol and almost one hour for acetaminophen. However, peak plasma concentration or the extent of absorption of either tramadol or acetaminophen were not affected. The clinical significance of this difference is unknown.
Distribution:
The volume of distribution of tramadol was 2.6 and 2.9 L/kg in male and female subjects, respectively, following a 100 mg intravenous dose. The binding of tramadol to human plasma proteins is approximately 20% and binding also appears to be independent of concentration up to 10 mcg/mL. Saturation of plasma protein bindings occurs only at concentrations outside the clinically relevant range.
Acetaminophen appears to be widely distributed throughout most body tissues except fat. Its apparent volume of distribution is about 0.9 L/kg. A relative small portion (~20%) of acetaminophen is bound to plasma protein.
Metabolism:
Following oral administration, tramadol is extensively metabolized by a number of pathways, including CYP2D6 and CYP3A4, as well as by conjugation of parent and metabolites. Approximately 30% of the dose is excreted in the urine as unchanged drug, whereas 60% of the dose is excreted as metabolites. The major metabolic pathways appear to be N- and O- demethylation and glucuronidation or sulfation in the liver. Metabolite M1 (O-desmethyltramadol) is pharmacologically active in animal models. Formation of M1 is dependent on CYP2D6 and as such is subject to inhibition, which may affect the therapeutic response (see PRECAUTIONS, Drug Interactions).
Approximately 7% of the population has reduced activity of the CYP2D6 isoenzyme of cytochrome P450. These individuals are “poor metabolizers" of debrisoquine, dextromethorphan, tricyclic antidepressants, among other drugs. Based on a population PK analysis of Phase 1 studies in healthy subjects, concentrations of tramadol were approximately 20% higher in "poor metabolizers" versus "extensive metabolizers," while M1 concentrations were 40% lower. In vitro drug interaction studies in human liver microsomes indicate that inhibitors of CYP2D6 such as fluoxetine and its metabolite norfluoxetine, amitriptyline and quinidine inhibit the metabolism of tramadol to various degrees. The full pharmacological impact of these alterations in terms of either efficacy or safety is unknown. Concomitant use of SEROTONIN re-uptake INHIBITORS and MAO INHIBITORS may enhance the risk of adverse events, including seizure (see WARNINGS) and serotonin syndrome.
Acetaminophen is primarily metabolized in the liver by first-order kinetics and involves three principal separate pathways:
a) conjugation with glucuronide;
b) conjugation with sulfate; and
c) oxidation via the cytochrome; P450-dependent, mixed-function oxidase enzyme pathway to form a reactive intermediate metabolite, which conjugates with glutathione and is then further metabolized to form cysteine and mercapturic acid conjugates. The principal cytochrome P450 isoenzyme involved appears to be CYP2E1, with CYP1A2 and CYP3A4 as additional pathways.
In adults, the majority of acetaminophen is conjugated with glucuronic acid and, to a lesser extent, with sulfate. These glucuronide-, sulfate-, and glutathione-derived metabolites lack biologic activity. In premature infants, newborns, and young infants, the sulfate conjugate predominates.
Elimination:
Tramadol is eliminated primarily through metabolism by the liver and the metabolites are eliminated primarily by the kidneys. The plasma elimination half-lives of racemic tramadol and M1 are approximately 5 to 6 and 7 hours, respectively, after administration of tramadol hydrochloride and acetaminophen tablets. The apparent plasma elimination half-life of racemic tramadol increased to 7 to 9 hours upon multiple dosing of tramadol hydrochloride and acetaminophen tablets.
The half-life of acetaminophen is about 2 to 3 hours in adults. It is somewhat shorter in children and somewhat longer in neonates and in cirrhotic patients. Acetaminophen is eliminated from the body primarily by formation of glucuronide and sulfate conjugates in a dose-dependent manner. Less than 9% of acetaminophen is excreted unchanged in the urine.
Special Populations
Renal:
The pharmacokinetics of tramadol hydrochloride and acetaminophen tablets in patients with renal impairment have not been studied. Based on studies using tramadol alone, excretion of tramadol and metabolite M1 is reduced in patients with creatinine clearance of less than 30 mL/min, adjustment of dosing regimen in this patient population is recommended. (See DOSAGE AND ADMINISTRATION.) The total amount of tramadol and M1 removed during a 4-hour dialysis period is less than 7% of the administered dose based on studies using tramadol alone.
Hepatic:
The pharmacokinetics and tolerability of tramadol hydrochloride and acetaminophen tablets in patients with impaired hepatic function has not been studied. Since tramadol and acetaminophen are both extensively metabolized by the liver, the use of tramadol hydrochloride and acetaminophen tablets in patients with hepatic impairment is not recommended (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Geriatric:
A population pharmacokinetic analysis of data obtained from a clinical trial in patients with chronic pain treated with tramadol hydrochloride and acetaminophen tablets which included 55 patients between 65 and 75 years of age and 19 patients over 75 years of age, showed no significant changes in pharmacokinetics of tramadol and acetaminophen in elderly patients with normal renal and hepatic function (see PRECAUTIONS, Geriatric Use).
-
CLINICAL STUDIES
Single Dose Studies for Treatment of Acute Pain
In pivotal single-dose studies in acute pain, two tablets of tramadol hydrochloride and acetaminophen administered to patients with pain following oral surgical procedures provided greater relief than placebo or either of the individual components given at the same dose. The onset of pain relief after tramadol hydrochloride and acetaminophen tablets was faster than tramadol alone. Onset of analgesia occurred in less than one hour. The duration of pain relief after tramadol hydrochloride and acetaminophen tablets was longer than acetaminophen alone. Analgesia was generally comparable to that of the comparator, ibuprofen.
- INDICATIONS AND USAGE
-
CONTRAINDICATIONS
Tramadol hydrochloride and acetaminophen tablets should not be administered to patients who have previously demonstrated hypersensitivity to tramadol, acetaminophen, any other component of this product or opioids. Tramadol hydrochloride and acetaminophen tablets are contraindicated in any situation where opioids are contraindicated, including acute intoxication with any of the following: alcohol, hypnotics, narcotics, centrally acting analgesics, opioids or psychotropic drugs. Tramadol hydrochloride and acetaminophen tablets may worsen central nervous system and respiratory depression in these patients.
-
WARNINGS
Seizure Risk
Seizures have been reported in patients receiving tramadol within the recommended dosage range. Spontaneous post-marketing reports indicate that seizure risk is increased with doses of tramadol above the recommended range. Concomitant use of tramadol increases the seizure risk in patients taking:
- Selective serotonin reuptake inhibitors (SSRI antidepressants or anorectics),
- Tricyclic antidepressants (TCAs), and other tricyclic compounds (e.g., cyclobenzaprine, promethazine, etc.), or
- Other opioids.
Administration of tramadol may enhance the seizure risk in patients taking:
- MAO inhibitors (see also WARNINGS - Use with MAO Inhibitors),
- Neuroleptics, or
- Other drugs that reduce the seizure threshold.
Risk of convulsions may also increase in patients with epilepsy, those with a history of seizures, or in patients with a recognized risk for seizure (such as head trauma, metabolic disorders, alcohol and drug withdrawal, CNS infections). In tramadol overdose, naloxone administration may increase the risk of seizure.
Anaphylactoid Reactions
Serious and rarely fatal anaphylactoid reactions have been reported in patients receiving therapy with tramadol. When these events do occur it is often following the first dose. Other reported allergic reactions include pruritus, hives, bronchospasm, angioedema, toxic epidermal necrolysis and Stevens-Johnson syndrome. Patients with a history of anaphylactoid reactions to codeine and other opioids may be at increased risk and therefore should not receive tramadol hydrochloride and acetaminophen tablets (see CONTRAINDICATIONS).
Respiratory Depression
Administer tramadol hydrochloride and acetaminophen tablets cautiously in patients at risk for respiratory depression. In these patients, alternative non-opioid analgesics should be considered. When large doses of tramadol are administered with anesthetic medications or alcohol, respiratory depression may result. Respiratory depression should be treated as an overdose. If naloxone is to be administered, use cautiously because it may precipitate seizures (see WARNINGS, Seizure Risk and OVERDOSAGE).
Interaction With Central Nervous System (CNS) Depressants
Tramadol hydrochloride and acetaminophen tablets should be used with caution and in reduced dosages when administered to patients receiving CNS depressants such as alcohol, opioids, anesthetic agents, narcotics, phenothiazines, tranquilizers or sedative hypnotics. Tramadol increases the risk of CNS and respiratory depression in these patients.
lncreased Intracranial Pressure or Head Trauma
Tramadol hydrochloride and acetaminophen tablets should be used with caution in patients with increased intracranial pressure or head injury. The respiratory depressant effects of opioids include carbon dioxide retention and secondary elevation of cerebrospinal fluid pressure and may be markedly exaggerated, in these patients. Additionally, pupillary changes (miosis) from tramadol may obscure the existence, extent or course of intracranial pathology. Clinicians should also maintain a high index of suspicion for adverse drug reaction when evaluating altered mental status in these patents if they are receiving tramadol hydrochloride and acetaminophen tablets (see Respiratory Depression).
Use In Ambulatory Patients
Tramadol may impair the mental and or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery. The patient using this drug should be cautioned accordingly.
Use With MAO Inhibitors and Serotonin Re-uptake Inhibitors
Use tramadol hydrochloride and acetaminophen tablets with great caution in patients taking monoamine oxidase inhibitors. Animal studies have shown increased deaths with combined administration of MAO inhibitors and tramadol. Concomitant use of tramadol with MAO inhibitors or SSRI's increases the risk of adverse events, including seizure and serotonin syndrome.
Use With Alcohol
Tramadol hydrochloride and acetaminophen tablets should not be used concomitantly with alcohol consumption. The use of tramadol hydrochloride and acetaminophen tablets in patients with liver disease is not recommended.
Use With Other Acetaminophen-containing Products
Due to the potential for acetaminophen hepatotoxicity at doses higher than the recommended dose, tramadol hydrochloride and acetaminophen tablets should not be used concomitantly with other acetaminophen-containing products.
Withdrawal
Withdrawal symptoms may occur if tramadol hydrochloride and acetaminophen tablets are discontinued abruptly. (See DRUG ABUSE AND DEPENDENCE.) These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Other symptoms that have been seen less frequently with tramadol hydrochloride and acetaminophen tablet discontinuation include: panic attacks, severe anxiety, and paresthesias. Clinical experience suggests that withdrawal symptoms may be avoided by tapering tramadol hydrochloride and acetaminophen tablets at the time of discontinuation.
Physical Dependence and Abuse
Tramadol may induce psychic and physical dependence of the morphine-type (µ-opioid). (See DRUG ABUSE AND DEPENDENCE.) Tramadol should not be used in opioid-dependent patients. Tramadol has been shown to reinitiate physical dependence in some patients that have been previously dependent on other opioids. Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug are not limited to those patients with prior history of opioid dependence.
Risk of Overdosage
Serious potential consequences of overdosage with tramadol are central nervous system depression, respiratory depression and death. In treating an overdose, primary attention should be given to maintaining adequate ventilation along with general supportive treatment. (See OVERDOSAGE.)
Serious potential consequences of overdosage with acetaminophen are hepatic (centrilobular) necrosis, leading to hepatic failure and death. Emergency help should be sought immediately and treatment initiated immediately if overdose is suspected, even if symptoms are not apparent.
-
PRECAUTIONS
General
The recommended dose of tramadol hydrochloride and acetaminophen tablets should not be exceeded.
Do not co-administer tramadol hydrochloride and acetaminophen tablets with other tramadol or acetaminophen-containing products. (See WARNINGS, Use With Other Acetaminophen-containing Products and Risk of Overdosage.)
Pediatric Use
The safety and effectiveness of tramadol hydrochloride and acetaminophen tablets has not been studied in the pediatric population.
Geriatric Use
In general, dose selection for an elderly patient should be cautious, reflecting the greater frequency of decreased hepatic, renal, or cardiac function; of concomitant disease and multiple drug therapy.
Acute Abdominal Conditions
The administration of tramadol hydrochloride and acetaminophen tablets may complicate the clinical assessment of patients with acute abdominal conditions.
Use In Renal Disease
Tramadol hydrochloride and acetaminophen tablets have not been studied in patients with impaired renal function. Experience with tramadol suggests that impaired renal function results in a decreased rate and extent of excretion of tramadol and its active metabolite, M1. In patients with creatinine clearances of less than 30 mL/min, it is recommended that the dosing interval of tramadol hydrochloride and acetaminophen tablets be increased not to exceed 2 tablets every 12 hours.
Use in Hepatic Disease
Tramadol hydrochloride and acetaminophen tablets have not been studied in patients with impaired hepatic function. The use of tramadol hydrochloride and acetaminophen tablets in patients with hepatic impairment is not recommended (see WARNINGS, Use With Alcohol).
Information for Patients
- Tramadol hydrochloride and acetaminophen tablets may impair mental or physical abilities required for the performance of potentially hazardous tasks such as driving a car or operating machinery.
- Tramadol hydrochloride and acetaminophen tablets should not be taken with alcohol containing beverages.
- The patient should be instructed not to take tramadol hydrochloride and acetaminophen tablets in combination with other tramadol or acetaminophen-containing products, including over-the-counter preparations.
- Tramadol hydrochloride and acetaminophen tablets should be used with caution when taking medications such as tranquilizers, hypnotics or other opiate containing analgesics.
- The patient should be instructed to inform the physician if they are pregnant, think they might become pregnant, or are trying to become pregnant (see PRECAUTIONS, Labor and Delivery).
- The patient should understand the single-dose and 24-hour dose limit and the time interval between doses, since exceeding these recommendations can result in respiratory depression, seizures, hepatic toxicity and death.
Drug Interactions
In vitro studies indicate that tramadol is unlikely to inhibit the CYP3A4-mediated metabolism of other drugs when tramadol is administered concomitantly at therapeutic doses. Tramadol does not appear to induce its own metabolism in humans, since observed maximal plasma concentrations after multiple oral doses are higher than expected based on single-dose data. Tramadol is a mild inducer of selected drug metabolism pathways measured in animals.
Use With Carbamazepine
Patients taking carbamazepine may have a significantly reduced analgesic effect of tramadol. Because carbamazepine increases tramadol metabolism and because of the seizure risk associated with tramadol, concomitant administration of tramadol hydrochloride and acetaminophen tablets and carbamazepine is not recommended.
Use With Quinidine
Tramadol is metabolized to M1 by CYP2D6. Quinidine is a selective inhibitor of that isoenzyme; so that concomitant administration of quinidine and tramadol results in increased concentrations of tramadol and reduced concentrations of M1. The clinical consequences of these findings are unknown. In vitro drug interaction studies in human liver microsomes indicate that tramadol has no effect on quinidine metabolism.
Use With Inhibitors of CYP2D6
In vitro drug interaction studies in human liver microsomes indicate that concomitant administration with inhibitors of CYP2D6 such as fluoxetine, paroxetine, and amitriptyline could result in some inhibition of the metabolism of tramadol.
Use With Cimetidine
Concomitant administration of tramadol hydrochloride and acetaminophen tablets and cimetidine has not been studied. Concomitant administration of tramadol and cimetidine does not result in clinically significant changes in tramadol pharmacokinetics. Therefore, no alteration of the tramadol hydrochloride and acetaminophen tablets dosage regimen is recommended.
Use With MAO Inhibitors
Interactions with MAO Inhibitors, due to interference with detoxification mechanisms, have been reported for some centrally acting drugs (see WARNINGS, Use With MAO Inhibitors).
Use With Digoxin
Post-marketing surveillance of tramadol has revealed rare reports of digoxin toxicity.
Use With Warfarin Like Compounds
Post-marketing surveillance of both tramadol and acetaminophen individual products have revealed rare alterations of warfarin effect, including elevation of prothrombin times.
While such changes have been generally of limited clinical significance for the individual products, periodic evaluation of prothrombin time should be performed when tramadol hydrochloride and acetaminophen tablets and warfarin-like compounds are administered concurrently.
Carcinogenesis, Mutagenesis, Impairment of Fertility
There are no animal or laboratory studies on the combination product (tramadol and acetaminophen) to evaluate carcinogenesis, mutagenesis, or impairment of fertility.
A slight but statistically significant increase in two common murine tumors, pulmonary and hepatic, was observed in a mouse carcinogenicity study, particularly in aged mice. Mice were dosed orally up to 30 mg/kg (90 mg/m2 or 0.5 times the maximum daily human tramadol dosage of 185 mg/m2) for approximately two years, although the study was not done with the Maximum Tolerated Dose. This finding is not believed to suggest risk in humans. No such finding occurred in rat carcinogenicity study (dosing orally up to 30 mg/kg, 180 mg/m2, or 1 time the maximum daily human tramadol dosage).
Tramadol was not mutagenic in the following assays: Ames Salmonella microsomal activation test, CHO/HPRT mammalian cell assay, mouse lymphoma assay (in the absence of metabolic activation), dominant lethal mutation tests in mice, chromosome aberration test in Chinese hamsters, and bone marrow micronucleus tests in mice and Chinese hamsters. Weakly mutagenic results occurred in the presence of metabolic activation in the mouse lymphoma assay and micronucleus test in rats. Overall, the weight of evidence from these tests indicates that tramadol does not pose a genotoxic risk to humans.
No effects on fertility were observed for tramadol at oral dose levels up to 50 mg/kg (350 mg/m2) in male rats and 75 mg/kg (450 mg/m2) in female rats. These dosages are 1.6 and 2.4 times the maximum daily human tramadol dosage of 185 mg/m2.
Pregnancy
Teratogenic Effects: Pregnancy Category C
No drug-related teratogenic effects were observed in the progeny of rats treated orally with tramadol and acetaminophen. The tramadol/acetaminophen combination product was shown to be embryotoxic and fetotoxic in rats at a maternally toxic dose, 50/434 mg/kg tramadol/acetaminophen (300/2604 mg/m2 or 1.6 times the maximum daily human tramadol/acetaminophen dosage of 185/1591 mg/m2), but was not teratogenic at this dose level. Embryo and fetal toxicity consisted of decreased fetal weights and increased supernumerary ribs.
Non-teratogenic effects:
Tramadol alone was evaluated in peri- and post-natal studies in rats. Progeny of dams receiving oral (gavage) dose levels of 50 mg/kg (300 mg/m2 or 1.6 times the maximum daily human tramadol dosage) or greater had decreased weights, and pup survival was decreased early in lactation at 80 mg/kg (480 mg/m2 or 2.6 times the maximum daily human tramadol dosage).
There are no adequate and well-controlled studies in pregnant women. Tramadol hydrochloride and acetaminophen tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Neonatal seizures, neonatal withdrawal syndrome, fetal death and still birth have been reported with tramadol hydrochloride during post-marketing.
Labor & Delivery
Tramadol hydrochloride and acetaminophen tablets should not be used in pregnant women prior to or during labor unless the potential benefits outweigh the risks. Safe use in pregnancy has not been established. Chronic use during pregnancy may lead to physical dependence and post-partum withdrawal symptoms in the newborn. (See DRUG ABUSE AND DEPENDENCE.) Tramadol has been shown to cross the placenta. The mean ratio of serum tramadol in the umbilical veins compared to maternal veins was 0.83 for 40 women given tramadol during labor.
The effect of tramadol hydrochloride and acetaminophen tablets, if any, on the later growth, development, and functional maturation of the child is unknown.
Nursing Mothers
Tramadol hydrochloride and acetaminophen tablets are not recommended for obstetrical preoperative medication or for post-delivery analgesia in nursing mothers because its safety in infants and newborns has not been studied.
Following a single IV 100 mg dose of tramadol, the cumulative excretion in breast milk within 16 hours post-dose was 100 mcg of tramadol (0.1% of the maternal dose) and 27 mcg of M1.
-
ADVERSE REACTIONS
Table 2 reports the incidence rate of treatment-emergent adverse events over five days of tramadol hydrochloride and acetaminophen tablet use in clinical trials (subjects took an average of at least 6 tablets per day).
Table 2: Incidence of Treatment-Emergent Adverse Events (≥2.0%) * Number of males = 62
Body System Tramadol Hydrochloride and Acetaminophen Tablets (N=142) % Preferred Term Gastrointestinal System Disorders Constipation 6 Diarrhea 3 Nausea 3 Dry Mouth 2 Psychiatric Disorders Somnolence 6 Anorexia 3 Insomnia 2 Central & Peripheral Nervous System Dizziness 3 Skin and Appendages Sweating Increased 4 Pruritus 2 Reproductive Disorders, Male[1] Prostatic Disorder 2 Incidence at least 1%, causal relationship at least possible or greater: the following lists adverse reactions that occurred with an incidence of at least 1% in single-dose or repeated-dose clinical trials of tramadol hydrochloride and acetaminophen tablets.
Body as a Whole - Asthenia, fatigue, hot flushes.
Central and Peripheral Nervous System - Dizziness, headache, tremor.
Gastrointestinal System - Abdominal pain, constipation, diarrhea, dyspepsia, flatulence, dry mouth, nausea, vomiting.
Psychiatric Disorders - Anorexia, anxiety, confusion, euphoria, insomnia, nervousness, somnolence.
Skin and Appendages - Pruritus, rash, increased sweating.
Selected Adverse events occurring at less than 1%:
The following lists clinically relevant adverse reactions that occurred with an incidence of less than 1% in tramadol hydrochloride and acetaminophen tablets clinical trials.
Body as a Whole - Chest pain, rigors, syncope, withdrawal syndrome.
Cardiovascular Disorders - Hypertension, aggravated hypertension, hypotension.
Central and Peripheral Nervous System - Ataxia, convulsions, hypertonia, migraine, aggravated migraine, involuntary muscle contractions, paraesthesia, stupor, vertigo.
Gastrointestinal System - Dysphagia, melena, tongue edema.
Hearing and Vestibular Disorders - Tinnitus.
Heart Rate and Rhythm Disorders - Arrhythmia, palpitation, tachycardia.
Liver and BiIiary System - Hepatic function abnormal.
Metabolic and Nutritional Disorders - Weight decrease.
Psychiatric Disorders - Amnesia, depersonalization, depression, drug abuse, emotional lability, hallucination, impotence, paroniria, abnormal thinking.
Red Blood Cell Disorders - Anemia.
Respiratory System - Dyspnea.
Urinary System - Albuminuria, micturition disorder, oliguria, urinary retention.
Vision Disorders - Abnormal vision.
Other clinically significant adverse experiences previously reported with tramadol hydrochloride.
Other events which have been reported with the use of tramadol products and for which a causal association has not been determined include: vasodilation, orthostatic hypotension, myocardial ischemia, pulmonary edema, allergic reactions (including anaphylaxis and urticaria, Stevens-Johnson syndrome/TENS), cognitive dysfunction, difficulty concentrating, depression, suicidal tendency, hepatitis liver failure and gastrointestinal bleeding. Reported laboratory abnormalities included elevated creatinine and liver function tests. Serotonin syndrome (whose symptoms may include mental status change, hyperreflexia, fever, shivering, tremor, agitation, diaphoresis, seizures and coma) has been reported with tramadol when used concomitantly with other serotonergic agents such as SSRIs and MAOIs.
Other clinically significant adverse experiences previously reported with acetaminophen.
Allergic reactions (primarily skin rash) or reports of hypersensitivity secondary to acetaminophen are rare and generally controlled by discontinuation of the drug and, when necessary, symptomatic treatment.
-
DRUG ABUSE AND DEPENDENCE
Tramadol may induce psychic and physical dependence of the morphine-type (µ-opioid). (See WARNINGS.) Dependence and abuse, including drug-seeking behavior and taking illicit actions to obtain the drug are not limited to those patients with a prior history of opioid dependence. The risk in patients with substance abuse has been observed to be higher. Tramadol is associated with craving and tolerance development. Withdrawal symptoms may occur if tramadol is discontinued abruptly. These symptoms may include: anxiety, sweating, insomnia, rigors, pain, nausea, tremors, diarrhea, upper respiratory symptoms, piloerection, and rarely hallucinations. Other symptoms that have been seen less frequently with tramadol hydrochloride and acetaminophen tablet discontinuation include: panic attacks, severe anxiety, and paresthesias. Clinical experience suggests that withdrawal symptoms may be relieved by reinstitution of opioid therapy followed by a gradual, tapered dose reduction of the medication combined with symptomatic support.
-
OVERDOSAGE
Tramadol hydrochloride and acetaminophen tablet is a combination product. The clinical presentation of overdose may include the signs and symptoms of tramadol toxicity, acetaminophen toxicity or both. The initial symptoms of tramadol overdosage may include respiratory depression and or seizures. The initial symptoms seen within the first 24 hours following an acetaminophen overdose are: anorexia, nausea, vomiting, malaise, pallor and diaphoresis.
Tramadol
Serious potential consequences of overdosage are respiratory depression, lethargy, coma, seizure, cardiac arrest and death. (See WARNINGS.) Fatalities have been reported in post marketing in association with both intentional and unintentional overdose with tramadol.
Acetaminophen
Serious potential consequences of overdosage with acetaminophen are hepatic centrilobular necrosis, leading to hepatic failure and death. Renal tubular necrosis, hypoglycemia and coagulation defects also may occur. Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post ingestion.
Treatment of Overdose
A single or multiple overdose with tramadol hydrochloride and acetaminophen tablets may be a potentially lethal polydrug overdose, and consultation with a regional poison control center is recommended.
In treating an overdose of tramadol hydrochloride and acetaminophen tablets, primary attention should be given to maintaining adequate ventilation along with general supportive treatment. While naloxone will reverse some, but not all, symptoms caused by overdosage with tramadol, the risk of seizures is also increased with naloxone administration. In animals, convulsions following the administration of toxic doses of tramadol could be suppressed with barbiturates or benzodiazepines but were increased with naloxone. Naloxone administration did not change the lethality of an overdose in mice. Based on experience with tramadol, hemodialysis is not expected to be helpful in an overdose because it removes less than 7% of the administered dose in a 4-hour dialysis period.
Standard recommendations should be followed for the treatment of acetaminophen overdose.
-
DOSAGE AND ADMINISTRATION
For the short-term (five days or less) management of acute pain, the recommended dose of tramadol hydrochloride and acetaminophen tablets, 37.5 mg/325 mg, is 2 tablets every 4 to 6 hours as needed for pain relief up to a maximum of 8 tablets per day.
Individualization of Dose
In patients with creatinine clearances of less than 30 mL/min, it is recommended that the dosing interval of tramadol hydrochloride and acetaminophen tablets, 37.5 mg/325 mg, be increased not to exceed 2 tablets every 12 hours. Dose selection for an elderly patient should be cautious, in view of the potential for greater sensitivity to adverse events.
-
HOW SUPPLIED
Tramadol hydrochloride and acetaminophen tablets, 37.5 mg/325 mg, (orange, film-coated capsule-shaped tablets) debossed "083" on one side and "KALI" on the other are available as follows:
Bottles of 20 tablets NDC: 21695-236-20 Bottles of 30 tablets NDC: 21695-236-30 Bottles of 50 tablets NDC: 21695-236-50 Bottles of 60 tablets NDC: 21695-236-60 Bottles of 120 tablets NDC: 21695-236-72 Dispense in a tight container. Store at 25oC (77oF); excursions permitted to 15º to 30oC (59º to 86oF).
Manufactured by:
PAR PHARMACEUTICAL COMPANIES, INC.
Spring Valley, NY 10977
Repackaged by:
REBEL DISTRIBUTORS CORP
Thousand Oaks, CA 91320
- PRINCIPAL DISPLAY PANEL – 37.5 MG/325 MG, 60 TABLETS
-
INGREDIENTS AND APPEARANCE
TRAMADOL HYDROCHLORIDE AND ACETAMINOPHEN
tramadol hydrochloride and acetaminophen tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 21695-236(NDC:49884-946) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TRAMADOL HYDROCHLORIDE (UNII: 9N7R477WCK) (TRAMADOL - UNII:39J1LGJ30J) TRAMADOL HYDROCHLORIDE 37.5 mg ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 325 mg Inactive Ingredients Ingredient Name Strength MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POVIDONE (UNII: FZ989GH94E) STARCH, PREGELATINIZED CORN (UNII: O8232NY3SJ) STEARIC ACID (UNII: 4ELV7Z65AP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) HYPROMELLOSE 2910 (3 MPA.S) (UNII: 0VUT3PMY82) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) HYPROMELLOSE 2910 (50 MPA.S) (UNII: 1IVH67816N) POLYDEXTROSE (UNII: VH2XOU12IE) POLYETHYLENE GLYCOL (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Product Characteristics Color ORANGE Score no score Shape OVAL (capsule-shaped) Size 15mm Flavor Imprint Code 083;KALI Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 21695-236-20 20 in 1 BOTTLE 2 NDC: 21695-236-30 30 in 1 BOTTLE 3 NDC: 21695-236-50 50 in 1 BOTTLE 4 NDC: 21695-236-60 60 in 1 BOTTLE 5 NDC: 21695-236-72 120 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA076475 04/21/2005 Labeler - Rebel Distributors Corp (118802834) Establishment Name Address ID/FEI Business Operations Rebel Distributors Corp 118802834 RELABEL, REPACK
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.