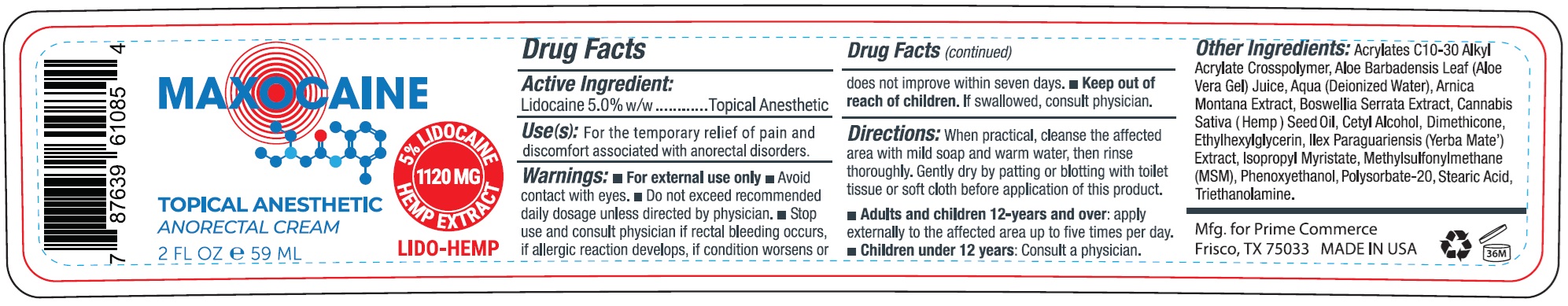
MAXOCAINE TOPICAL ANESTHETIC ANORECTAL- lidocaine cream
MAXOCAINE Topical Anesthetic Anorectal by
Drug Labeling and Warnings
MAXOCAINE Topical Anesthetic Anorectal by is a Otc medication manufactured, distributed, or labeled by PRIME COMMERCE, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredient:
- Use(s):
- Warnings:
-
Directions:
When practical, cleanse the affected area with mild soap and warm water, then rinse thoroughly. Gently dry by patting or blotting with toilet tissue or soft cloth before application of this product.
- Adults and children 12-years and over:apply externally to the affected area up to five times per day.
- Children under 12-years:Consult a physician.
-
Other Ingredients:
Acrylates C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis Leaf (Aloe Vera Gel) Juice, Aqua (Deionzied Water), Arnica Montana Extract, Boswellia Serrata Extract, Cannabis Sativa (Hemp) Seed Oil, Cetyl Alcohol, Dimethicone, Ethylhexylglycerin, Ilex Paraguariensis (Yerba Mate) Extract, Isopropyl Myristate, methylsulfonylmethane (MSM), Phenoxyethanol, Polysorbate-20, Stearic Acid, Triethanolamine.
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
MAXOCAINE TOPICAL ANESTHETIC ANORECTAL
lidocaine creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72188-076 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) ALOE VERA LEAF (UNII: ZY81Z83H0X) WATER (UNII: 059QF0KO0R) ARNICA MONTANA WHOLE (UNII: O80TY208ZW) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE (UNII: 92RU3N3Y1O) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ILEX PARAGUARIENSIS LEAF (UNII: 1Q953B4O4F) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) STEARIC ACID (UNII: 4ELV7Z65AP) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72188-076-59 59 mL in 1 JAR; Type 0: Not a Combination Product 05/15/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M015 05/15/2025 Labeler - PRIME COMMERCE, LLC (016785608) Registrant - Pure Source, LLC (080354456)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.