Senna S by Care One (American Sales Company) / P & L Development, LLC DRUG FACTS
Senna S by
Drug Labeling and Warnings
Senna S by is a Otc medication manufactured, distributed, or labeled by Care One (American Sales Company), P & L Development, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
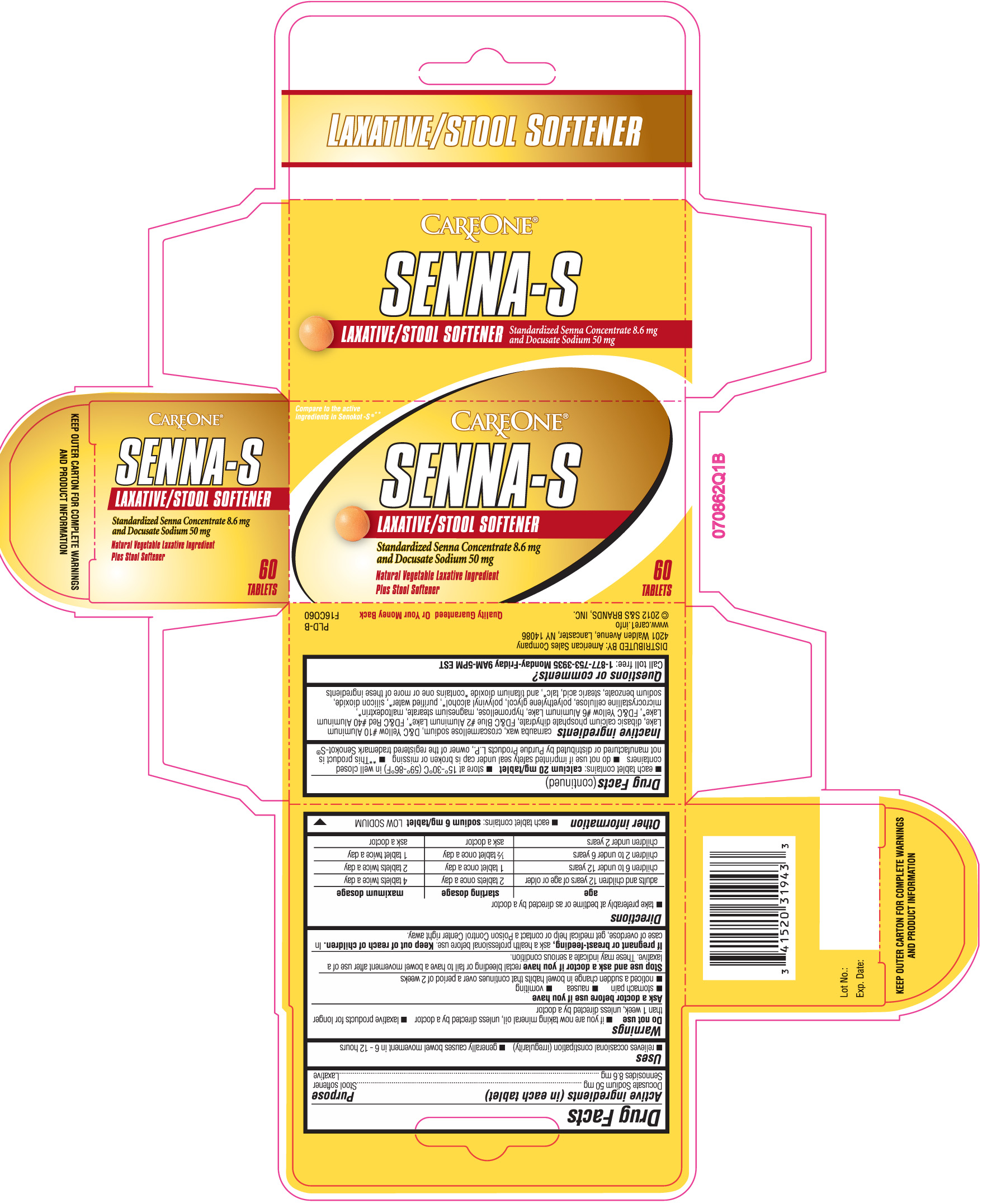
SENNA S- docusate sodium and sennosides tablet
Care One (American Sales Company)
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
DRUG FACTS
Do not use
- if you are now taking mineral oil, unless directed by a doctor
- laxative products for longer than 1 week unless directed by a doctor
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowel habits that continues over a period of 2 weeks
Stop use and ask a doctor if
you have rectal bleeding or fail to have a bowel movement after use of a laxative. These may indicate a serious condition.
Keep out of reach of children.
In case of overdose, get medical help or contact a Poison Control Center right away.
Directions
- take preferably at bedtime or as directed by a doctor
| age | starting dosage | maximum dosage |
| adults and children 12 years of age or older | 2 tablets once a day | 4 tablets twice a day |
| children 6 to under 12 years | 1 tablet once a day | 2 tablets twice a day |
| children 2 to under 6 years | 1/2 tablet once a day | 1 tablet twice a day |
| children under 2 years | ask a doctor | ask a doctor |
Other Information
- each tablet contains: sodium 6 mg/tablet LOW SODIUM
- each tablet contains: Calcium 20 mg/tablet
- store at 15o- 30o C (59o-86o F) in well closed containers
- do not use if imprinted safety seal under cap is broken or missing
- **This product is not manufactured or distributed by Purdue products L.P., owner of the registered trademark Senokot-S®
Inactive ingredients
carnauba wax, croscarmellose sodium, D&C yellow #10 aluminum lake, dibasic calcium phosphate dihydrate, FD&C blue #2 aluminum lake*, FD&C red #40 aluminum lake*, FD&C yellow #6 aluminum lake, hypromellose, magnesium stearate, maltodextrin*, microcrystalline cellulose, polyethylene glycol, polyvinyl alcohol*, purified water*, silicon dioxide, sodium benzoate, stearic acid, talc*, titanium dioxide
*contains one or more of these ingredients
Principal Display Panel
Compare to the active ingredients in Senokot- S® **
SENNA-S
Laxative/ Stool softener
Standardized senna concentrate 8.6 mg and Docusate Sodium 50 mg
Natural vegetable laxative ingredient plus stool softener
DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
Distributed by: American Sales Company
4201 Walden avenue, Lancaster, NY 14086
| SENNA S
docusate sodium and sennosides tablet |
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Labeler - Care One (American Sales Company) (809183973) |
| Registrant - P & L Development, LLC (800014821) |