DOXYCYCLINE HYCLATE- doxyclycline hyclate tablet, coated
DOXYCYCLINE HYCLATE by
Drug Labeling and Warnings
DOXYCYCLINE HYCLATE by is a Prescription medication manufactured, distributed, or labeled by EPIC PHARMA, LLC, Caribe Holdings (Cayman) Co. Ltd. dba PuraCap Caribe. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DOXYCYCLINE HYCLATE TABLETS safely and effectively. See full prescribing information for DOXYCYCLINE HYCLATE TABLETS.
Initial U.S. Approval: 1967INDICATIONS AND USAGE
Doxycycline hyclate tablets are tetracycline class drugs indicated for:
- Rickettsial infections (1.1)
- Sexually transmitted infections (1.2)
- Respiratory tract infections (1.3)
- Specific bacterial infections (1.4)
- Ophthalmic infections (1.5)
- Anthrax, including inhalational anthrax (post-exposure) (1.6)
- Alternative treatment for selected infections when penicillin is contraindicated (1.7)
- Adjunctive therapy for acute intestinal amebiasis and severe acne (1.8)
- Prophylaxis of malaria (1.9)
To reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline hyclate tablets and other antibacterial drugs, doxycycline hyclate tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria. (1.10)
DOSAGE AND ADMINISTRATION
- Important Administration Instructions for Doxycycline Hyclate Tablets
- o Doxycycline hyclate tablets (150 mg) can be broken into two-thirds or one-third to provide a 50 mg and 100 mg strength, respectively. (2.1)
- Dosage in Adults for Doxycycline Hyclate Tablets
- o The usual dosage is 200 mg on the first day of treatment (administered 100 mg every 12 hours) followed by a maintenance dose of 100 mg daily. (2.1)
- o In the management of more severe infections (particularly chronic infections of the urinary tract), 100 mg every 12 hours is recommended. (2.1)
- Dosage in Pediatric Patients for Doxycycline Hyclate Tablets
- o For all pediatric patients weighing less than 45 kg with severe or life-threatening infections (e.g., anthrax, Rocky Mountain spotted fever), the recommended dose is 2.2 mg per kg of body weight administered every 12 hours. Pediatric patients weighing 45 kg or more should receive the adult dose. (2.3)
- o For pediatric patients with less severe disease (greater than 8 years of age and weighing less than 45 kg), the recommended dose is 4.4 mg per kg of body weight divided into two doses on the first day of treatment, followed by a maintenance dose of 2.2 mg per kg of body weight (given as a single daily dose or divided into two doses. For pediatric patients weighing over 45 kg, the usual adult dose should be used. (2.3)
- See Full Prescribing Information for additional indication specific dosage information and important administration instructions for doxycycline hyclate tablets. (2.1, 2.4, 2.5)
DOSAGE FORMS AND STRENGTHS
Doxycycline Hyclate Tablets, USP: 75 mg and 150 mg (functionally scored) (3)
CONTRAINDICATIONS
Doxycycline hyclate tablets are contraindicated in persons who have shown hypersensitivity to any of the tetracyclines. (4)
WARNINGS AND PRECAUTIONS
- The use of doxycycline hyclate tablets during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown) and enamel hypoplasia Advise the patient of the potential risk to the fetus during pregnancy. (2.2, 5.1, 8.1, 8.4)
- The use of doxycycline hyclate tablets during the second and third-trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth. Advise the patient of the potential risk to the fetus during pregnancy. (5.2, 8.1, 8.4)
- Clostridioides difficile-associated diarrhea (CDAD) has been reported. Evaluate patients if diarrhea occurs. (5.3)
- Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Limit sun exposure. (5.4)
- Overgrowth of non-susceptible organisms, including fungi, may occur. If such infections occur, discontinue use and institute appropriate therapy. (5.10)
ADVERSE REACTIONS
Adverse reactions observed in patients receiving tetracyclines include anorexia, nausea, vomiting, diarrhea, rash, photosensitivity, urticaria, and hemolytic anemia. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Epic Pharma, LLC at 1-888-374-2791 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- Patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage. (7.1)
- Avoid co-administration of tetracyclines with penicillin. (7.2)
- Absorption of tetracyclines, including doxycycline hyclate tablets are impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate and iron-containing preparations. (7.3)
- Concurrent use of tetracyclines, including doxycycline hyclate tablets may render oral contraceptives less effective. (7.4)
- Barbiturates, carbamazepine and phenytoin decrease the half-life of doxycycline. (7.5)
USE IN SPECIFIC POPULATIONS
- Lactation: Breastfeeding is not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 9/2023
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
1.1 Rickettsial Infections
1.2 Sexually Transmitted Infections
1.3 Respiratory Tract Infections
1.4 Specific Bacterial Infections
1.5 Ophthalmic Infections
1.6 Anthrax Including Inhalational Anthrax (Post-Exposure)
1.7 Alternative Treatment for Selected Infections when Penicillin is Contraindicated
1.8 Adjunctive Therapy for Acute Intestinal Amebiasis and Severe Acne
1.9 Prophylaxis of Malaria
1.10 Usage
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage in Adult Patients
2.3 Dosing in Pediatric Patients
2.4 Dosage for Prophylaxis of Malaria
2.5 Dosage for Inhalational Anthrax (Post-Exposure)
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Tooth Development
5.2 Inhibition of Bond Growth
5.3 Clostridioides difficile Associated Diarrhea
5.4 Photosensitivity
5.5 Severe Skin Reactions
5.6 Intracranial Hypertension
5.7 Antianabolic Action
5.8 Incomplete Suppression of Malaria
5.9 Development of Drug-Resistant Bacteria
5.10 Potential for Microbial Overgrowth
5.11 Laboratory Monitoring for Long-Term Therapy
6 ADVERSE REACTIONS
7 DRUG INTERACTIONS
7.1 Anticoagulant Drugs
7.2 Penicillin
7.3 Antacids and Iron Preparations
7.4 Oral Contraceptives
7.5 Barbiturates and Anti-Epileptics
7.6 Penthrane®
7.7 Drug and Laboratory Test Interactions
8 USE IN SPECIFIC POPULATIONS
8.2 Lactation
8.3 Females and Males of Reproductive Potential
8.4 Pediatric Use
8.5 Geriatric Use
10 OVERDOSAGE
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
12.4 Microbiology
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
15 REFERENCES
16 HOW SUPPLIED/STORAGE AND HANDLING
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
1.1 Rickettsial Infections
Doxycycline hyclate tablets are indicated for treatment of Rocky Mountain spotted fever, typhus fever and the typhus group, Q fever, rickettsial pox, and tick fevers caused by Rickettsiae.
1.2 Sexually Transmitted Infections
Doxycycline hyclate tablets are indicated for treatment of the following sexually transmitted infections:
- Uncomplicated urethral, endocervical or rectal infections caused by Chlamydia trachomatis.
- Nongonococcal urethritis caused by Ureaplasma urealyticum.
- Lymphogranuloma venereum caused by Chlamydia trachomatis.
- Granuloma inguinale caused by Klebsiella granulomatis.
- Uncomplicated gonorrhea caused by Neisseria gonorrhoeae.
Chancroid caused by Haemophilus ducreyi.
1.3 Respiratory Tract Infections
Doxycycline hyclate tablets are indicated for treatment of the following respiratory tract infections:
- Respiratory tract infections caused by Mycoplasma pneumoniae.
- Psittacosis (ornithosis) caused by Chlamydophila psittaci.
- Because many strains of the following groups of microorganisms have been shown to be resistant to doxycycline, culture and susceptibility testing are recommended.
- Doxycycline is indicated for treatment of infections caused by the following microorganisms, when bacteriological testing indicates appropriate susceptibility to the drug.
- Respiratory tract infections caused by Haemophilus influenzae.
- Respiratory tract infections caused by Klebsiella species.
Upper respiratory infections caused by Streptococcus pneumoniae.
1.4 Specific Bacterial Infections
Doxycycline hyclate tablets are indicated for treatment of the following specific bacterial infections:
- Relapsing fever due to Borrelia recurrentis.
- Plague due to Yersinia pestis.
- Tularemia due to Francisella tularensis.
- Cholera caused by Vibrio cholerae.
- Campylobacter fetus infections caused by Campylobacter fetus.
- Brucellosis due to Brucella species (in conjunction with streptomycin).
- Bartonellosis due to Bartonella bacilliformis.
Because many strains of the following groups of microorganisms have been shown to be resistant to doxycycline, culture and susceptibility testing are recommended.
Doxycycline hyclate tablets are indicated for treatment of infections caused by the following gram-negative microorganisms, when bacteriological testing indicates appropriate susceptibility to the drug:
- Escherichia coli
- Enterobacter aerogenes
- Shigella species
- Acinetobacter species
Urinary tract infections caused by Klebsiella species.
1.5 Ophthalmic Infections
Doxycycline hyclate tablets are indicated for treatment of the following ophthalmic infections:
- Trachoma caused by Chlamydia trachomatis, although the infectious agent is not always eliminated as judged by immunofluorescence.
Inclusion conjunctivitis caused by Chlamydia trachomatis.
1.6 Anthrax Including Inhalational Anthrax (Post-Exposure)
Doxycycline hyclate tablets are indicated for the treatment of Anthrax due to Bacillus anthracis, including inhalational anthrax (post-exposure); to reduce the incidence or progression of disease following exposure to aerosolized Bacillus anthracis.
1.7 Alternative Treatment for Selected Infections when Penicillin is Contraindicated
Doxycycline hyclate tablets are indicted as an alternative treatment for the following selected infections when penicillin is contraindicated:
- Syphilis caused by Treponema pallidum.
- Yaws caused by Treponema pallidum subspecies pertenue.
- Listeriosis due to Listeria monocytogenes.
- Vincent’s infection caused by Fusobacterium fusiforme.
- Actinomycosis caused by Actinomyces israelii.
Infections caused by Clostridium species.
1.8 Adjunctive Therapy for Acute Intestinal Amebiasis and Severe Acne
In acute intestinal amebiasis, doxycycline hyclate tablets may be a useful adjunct to amebicides.
In severe acne, doxycycline hyclate tablets may be useful adjunctive therapy.
1.9 Prophylaxis of Malaria
Doxycycline hyclate tablets are indicated for the prophylaxis of malaria due to Plasmodium falciparum in short-term travelers (less than 4 months) to areas with chloroquine and/or pyrimethaminesulfadoxine resistant strains [see Dosage and Administration (2.4) and Patient Counseling Information (17)].
1.10 Usage
To reduce the development of drug-resistant bacteria and maintain the effectiveness of doxycycline hyclate tablets and other antibacterial drugs, doxycycline hyclate tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
- The usual dosage and frequency of administration of doxycycline hyclate tablets differ from that of the other tetracyclines. Exceeding the recommended dosage may result in an increased incidence of adverse reactions.
- Administer doxycycline hyclate tablets with adequate amounts of fluid to wash down the drugs and reduce the risk of esophageal irritation and ulceration [see Adverse Reactions (6)].
- If gastric irritation occurs, doxycycline hyclate tablets may be given with food or milk [see Clinical Pharmacology (12.3)].
- Doxycycline hyclate tablets (150 mg) can be broken into two-thirds or one-third to provide a 100 mg and 50 mg strength, respectively [see FDA-approved patient labeling].
2.2 Dosage in Adult Patients
- The usual dosage of doxycycline hyclate tablets are 200 mg on the first day of treatment (administered 100 mg every 12 hours) followed by a maintenance dose of 100 mg daily. The maintenance dose may be administered as a single dose or as 50 mg every 12 hours.
- In the management of more severe infections (particularly chronic infections of the urinary tract), 100 mg every 12 hours is recommended.
- For certain selected specific indications, the recommended duration or dosage and duration of doxycycline hyclate tablets in adult patients are as follows:
- 1. Streptococcal infections, therapy should be continued for 10 days.
- 2. Uncomplicated urethral, endocervical, or rectal infection caused by Chlamydia trachomatis: 100 mg by mouth twice-a-day for 7 days.
- 3. Uncomplicated gonococcal infections in adults (except anorectal infections in men): 100 mg, by mouth, twice-a-day for 7 days. As an alternate single visit dose, administer 300 mg stat followed in one hour by a second 300 mg dose.
- 4. Nongonococcal urethritis (NGU) caused by C. trachomatis and U. urealyticum: 100 mg by mouth twice-a-day for 7 days.
- 5. Syphilis – early: Patients who are allergic to penicillin should be treated with doxycycline 100 mg by mouth twice-a-day for 2 weeks.
- 6. Syphilis of more than one year’s duration: Patients who are allergic to penicillin should be treated with doxycycline 100 mg by mouth twice-a-day for 4 weeks.
- 7. Acute epididymo-orchitis caused by N. gonorrhoeae: 100 mg by mouth, twice-a-day for at least 10 days.
- 8. Acute epididymo-orchitis caused by C. trachomatis: 100 mg, by mouth, twice-a-day for at least 10 days.
2.3 Dosing in Pediatric Patients
- For all pediatric patients weighing less than 45 kg with severe or life threatening infections (e.g., anthrax, Rocky Mountain spotted fever), the recommended dosage of doxycycline hyclate tablets are 2.2 mg per kg of body weight administered every 12 hours. Pediatric patients weighing 45 kg or more should receive the adult dose [see Warnings and Precautions (5.1)].
- For pediatric patients with less severe disease (greater than 8 years of age and weighing less than 45 kg), the recommended dosage schedule of doxycycline hyclate tablets are 4.4 mg per kg of body weight divided into two doses on the first day of treatment, followed by a maintenance dose of 2.2 mg per kg of body weight (given as a single daily dose or divided into twice daily doses). For pediatric patients weighing over 45 kg, the usual adult dose should be used.
2.4 Dosage for Prophylaxis of Malaria
For adults, the recommended dose of doxycycline hyclate tablets are 100 mg daily.
For pediatric patients 8 years of age and older, the recommended dosage of doxycycline hyclate tablets are 2 mg per kg of body weight administered once daily. Pediatric patients weighing 45 kg or more should receive the adult dose.
Prophylaxis should begin 1 or 2 days before travel to the malarious area. Prophylaxis should be continued daily during travel in the malarious area and for 4 weeks after the traveler leaves the malarious area.
2.5 Dosage for Inhalational Anthrax (Post-Exposure)
For adults, the recommended dosage is 100 mg, of doxycycline hyclate tablets, by mouth, twice-a-day for 60 days.
For pediatric patients weighing less than 45 kg, the recommended dosage of doxycycline hyclate tablets are 2.2 mg per kg of body weight, by mouth, twice-a-day for 60 days. Pediatric patients weighing 45 kg or more should receive the adult dose.
-
3 DOSAGE FORMS AND STRENGTHS
Doxycycline Hyclate Tablets, USP 75 mg are round blue film coated tablets, debossed “PC90” on one side and blank on the other side. (each tablet contains 75 mg doxycycline as 86.6 mg of doxycycline hyclate).
Doxycycline Hyclate Tablets USP, 150 mg are capsule shaped green film coated tablets. Each side of the functionally scored tablet has two parallel score lines for splitting into 3 equal portions with “P” debossed on far-left, and “C” debossed on far-right portion of one side of the tablet, and “9” debossed on far-left and “1” debossed on far-right on the other side of the tablet. (each tablet contains 150 mg doxycycline as 173.2 mg of doxycycline hyclate).
- 4 CONTRAINDICATIONS
-
5 WARNINGS AND PRECAUTIONS
5.1 Tooth Development
The use of doxycycline hyclate during tooth development (last half of pregnancy, infancy and childhood to the age of 8 years) may cause permanent discoloration of the teeth (yellow-gray-brown). This adverse reaction is more common during long-term use of the drugs of the tetracycline class, but it has been observed following repeated short-term courses. Enamel hypoplasia has also been reported with drugs of the tetracycline class. Advise the patient of the potential risk to the fetus if doxycycline hyclate is used during pregnancy [see Use in Specific Populations (8.1, 8.4)].
Use doxycycline hyclate in pediatric patients 8 years of age or less only when the potential benefits are expected to outweigh the risks in severe or life-threatening conditions (e.g., anthrax, Rocky Mountain spotted fever), particularly when there are no alternative therapies.
5.2 Inhibition of Bond Growth
The use of doxycycline hyclate during the second and third trimester of pregnancy, infancy and childhood up to the age of 8 years may cause reversible inhibition of bone growth. All tetracyclines form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued. Advise the patient of the potential risk to the fetus if doxycycline hyclate is used during pregnancy [see Use in Specific Populations (8.1, 8.4)].
5.3 Clostridioides difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including doxycycline hyclate, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antibacterial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibacterial use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibacterial treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
5.4 Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines. Patients apt to be exposed to direct sunlight or ultraviolet light should be advised that this reaction can occur with tetracycline drugs, and treatment should be discontinued at the first evidence of skin erythema.
5.5 Severe Skin Reactions
Severe skin reactions, such as exfoliative dermatitis, erythema multiforme, Stevens-Johnson syndrome, toxic epidermal necrolysis, and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported in patients receiving doxycycline [See Adverse Reactions (6)]. If severe skin reactions occur, doxycycline should be discontinued immediately and appropriate therapy should be instituted.
5.6 Intracranial Hypertension
Intracranial hypertension (IH, pseudotumor cerebri) has been associated with the use of tetracyclines including doxycycline hyclate. Clinical manifestations of IH include headache, blurred vision, diplopia, and vision loss; papilledema can be found on fundoscopy. Women of childbearing age who are overweight or have a history of IH are at greater risk for developing tetracycline associated IH. Concomitant use of isotretinoin and doxycycline hyclate should be avoided because isotretinoin is also known to cause pseudotumor cerebri.
Although IH typically resolves after discontinuation of treatment, the possibility for permanent visual loss exists. If visual disturbance occurs during treatment, prompt ophthalmologic evaluation is warranted. Since intracranial pressure can remain elevated for weeks after drug cessation patients should be monitored until they stabilize.
5.7 Antianabolic Action
The antianabolic action of the tetracyclines may cause an increase in BUN. Studies to date indicate that this does not occur with the use of doxycycline in patients with impaired renal function.
5.8 Incomplete Suppression of Malaria
Doxycycline offers substantial but not complete suppression of the asexual blood stages of Plasmodium strains.
Doxycycline does not suppress P. falciparum’s sexual blood stage gametocytes. Subjects completing this prophylactic regimen may still transmit the infection to mosquitoes outside endemic areas.
5.9 Development of Drug-Resistant Bacteria
Prescribing doxycycline hyclate in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
-
6 ADVERSE REACTIONS
The following adverse reactions have been identified during clinical trials or post-approval use of tetracycline-class drugs, including doxycycline. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal: Anorexia, nausea, vomiting, diarrhea, glossitis, dysphagia, enterocolitis, inflammatory lesions (with monilial overgrowth) in the anogenital region, and pancreatitis. Hepatotoxicity has been reported. These reactions have been caused by both the oral and parenteral administration of tetracyclines. Superficial discoloration of the adult permanent dentition, reversible upon drug discontinuation and professional dental cleaning has been reported. Permanent tooth discoloration and enamel hypoplasia may occur with drugs of the tetracycline class when used during tooth development [See Warnings and Precautions (5.1)]. Instances of esophagitis and esophageal ulcerations have been reported in patients receiving capsule and tablet forms of drugs in the tetracycline-class. Most of these patients took medications immediately before going to bed [see Dosage and Administration (2.1)].
Skin: Maculopapular and erythematous rashes, Stevens-Johnson syndrome, toxic epidermal necrolysis, exfoliative dermatitis, and erythema multiforme have been reported. Photosensitivity has been reported [see Warnings and Precautions (5.4)].
Renal: Rise in BUN has been reported and is apparently dose-related [see Warnings and Precautions (5.7)].
Hypersensitivity reactions: Urticaria, angioneurotic edema, anaphylaxis, anaphylactoid purpura, serum sickness, pericarditis, exacerbation of systemic lupus erythematosus, and drug reaction with eosinophilia and systemic symptoms (DRESS).
Blood: Hemolytic anemia, thrombocytopenia, neutropenia, and eosinophilia have been reported.
Intracranial Hypertension: Intracranial hypertension (IH, pseudotumor cerebri) has been associated with the use of tetracyclines [see Warnings and Precautions (5.6)].
Thyroid Gland Changes: When given over prolonged periods, tetracyclines have been reported to produce brown-black microscopic discoloration of thyroid glands. No abnormalities of thyroid function are known to occur.
-
7 DRUG INTERACTIONS
7.1 Anticoagulant Drugs
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
7.2 Penicillin
Since bacteriostatic drugs may interfere with the bactericidal action of penicillin, it is advisable to avoid giving tetracyclines, including doxycycline hyclate in conjunction with penicillin.
7.3 Antacids and Iron Preparations
Absorption of tetracyclines, including doxycycline hyclate is impaired by antacids containing aluminum, calcium, or magnesium, bismuth subsalicylate, and iron-containing preparations.
7.4 Oral Contraceptives
Concurrent use of tetracyclines, including doxycycline hyclate may render oral contraceptives less effective.
7.5 Barbiturates and Anti-Epileptics
Barbiturates, carbamazepine, and phenytoin decrease the half-life of doxycycline.
-
8 USE IN SPECIFIC POPULATIONS
8.2 Lactation
Risk Summary
Based on available published data, doxycycline is present in human milk. There are no data that inform the levels of doxycycline in breastmilk, the effects on the breastfed infant, or the effects on milk production. Because of the potential for serious adverse reactions, including tooth discoloration and inhibition of bone growth, advise patients that breastfeeding is not recommended during treatment with doxycycline hyclate and for 5 days after the last dose.
8.3 Females and Males of Reproductive Potential
Infertility
Based on findings from a fertility study in animals, doxycycline may impair female and male fertility. The reversibility of this finding is unclear. [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
Because of the effects of drugs of the tetracycline-class on tooth development and growth, use doxycycline hyclate in pediatric patients 8 years of age or less only when the potential benefits are expected to outweigh the risks in severe or life-threatening conditions (e.g., anthrax, Rocky Mountain spotted fever), particularly when there are no alternative therapies [see Warnings and Precautions (5.1, 5.11) and Dosage and Administration (2.1, 2.5)].
8.5 Geriatric Use
Clinical studies of doxycycline hyclate tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
Doxycycline hyclate tablets each contains less than 1 mg of sodium.
- 10 OVERDOSAGE
-
11 DESCRIPTION
Doxycycline Hyclate Tablets, USP contain doxycycline hyclate, USP a tetracycline class drug synthetically derived from oxytetracycline, in an immediate release formulation for oral administration.
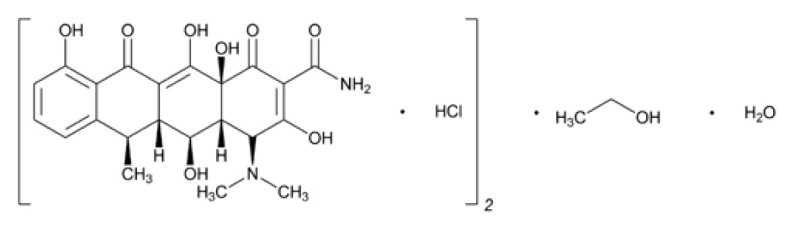
The molecular formula of doxycycline hyclate is (C22H24N2O8 · HCl)2 ·C2H6O·H2O and the molecular weight of doxycycline hyclate is 1025.87. The chemical name for doxycycline hyclate is: 4-(Dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,5,10,12,12a-pentahydroxy-6-methyl-1,11-dioxo-2 naphthacenecarboxamide monohydrochloride, compound with ethyl alcohol (2:1), monohydrate.
The structural formula for doxycycline hyclate is:
Figure 1: Structure of Doxycycline Hyclate
Doxycycline hyclate is a yellow crystalline powder soluble in water and in solutions of alkali hydroxides and carbonates.
Doxycycline hyclate tablets, USP are available as 75 mg and 150 mg tablets. Each 75 mg tablet contains 86.6 mg of doxycycline hyclate equivalent to 75 mg of doxycycline. Each 150 mg tablet contains 173.2 mg of doxycycline hyclate equivalent to 150 mg of doxycycline.
Inactive ingredients in the tablet formulation are: carnauba wax, croscarmellose sodium, FD&C Blue #1 Aluminum Lake (75 mg Tablet), FD&C Blue #2 Aluminum Lake (150 mg Tablet), FD&C Yellow #6 Aluminum Lake (75 mg Tablet), iron oxide yellow (150 mg Tablet), macrogol/PEG, magnesium stearate, NF (vegetable grade), microcrystalline cellulose, polyvinyl alcohol-part hydrolyzed, purified water, sodium lauryl sulfate, talc, titanium dioxide. Doxycycline hyclate tablets USP, 75 mg contain 0.128 mg (0.0056 mEq) of sodium. Doxycycline hyclate tablets USP, 150 mg contain 0.256 mg (0.0111 mEq) of sodium.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Doxycycline is a tetracycline-class antimicrobial drug [see Microbiology (12.4)].
12.3 Pharmacokinetics
Absorption
Doxycycline hyclate tablets: Following administration of a single 300 mg dose to adult volunteers, average peak plasma doxycycline levels were 3.0 mcg per mL at 3 hours, decreasing to 1.18 mcg per mL at 24 hours. The mean Cmax and AUC0-∞ of doxycycline are 24% and 15% lower, respectively, following single dose administration of doxycycline hyclate tablets, 150 mg tablets with a high fat meal (including milk) compared to fasted conditions. The clinical significance of these decreases is unknown.
Excretion
Tetracyclines are concentrated in bile by the liver and excreted in the urine and feces at high concentrations and in a biologically active form.
Excretion of doxycycline by the kidney is about 40% per 72 hours in individuals with a creatinine clearance of about 75 mL per minute. This percentage may fall as low as 1% per 72 hours to 5% per 72 hours in individuals with a creatinine clearance below 10 mL per minute. Studies have shown no significant difference in the serum half-life of doxycycline (range 18 to 22 hours) in individuals with normal and severely impaired renal function. Hemodialysis does not alter the serum half-life.
Pediatric Patients
Population pharmacokinetic analysis of sparse concentration-time data of doxycycline following standard of care intravenous and oral dosing in 44 children (2-18 years of age) showed that allometrically-scaled clearance of doxycycline in children ≥2 to ≤8 years of age (median [range] 3.58 [2.27-10.82] L/h/70 kg, N=11) did not differ significantly from children >8 to 18 years of age (3.27 [1.11-8.12] L/h/70 kg, N=33). For pediatric patients weighing ≤45 kg, body weight normalized doxycycline CL in those ≥2 to ≤8 years of age (median [range] 0.071 [0.041-0.202] L/kg/h, N=10) did not differ significantly from those >8 to 18 years of age (0.081 [0.035-0.126] L/kg/h, N=8). In pediatric patients weighing >45 kg no clinically significant differences in body weight normalized doxycycline CL were observed between those ≥2 to ≤8 years (0.050 L/kg/h, N=1) and those >8 years of age (0.044 [0.014-0.121] L/kg/h, N=25). No clinically significant difference in CL differences between oral and IV were observed in the small cohort of pediatric patients who received the oral (N=19) or IV (N=21) formulation alone.
12.4 Microbiology
Mechanism of Action
Doxycycline inhibits bacterial protein synthesis by binding to the 30S ribosomal subunit. Doxycycline has bacteriostatic activity against a broad range of Gram-positive and Gram-negative bacteria.
Resistance
Cross resistance with other tetracyclines is common.
Antimicrobial Activity
Doxycycline has been shown to be active against most isolates of the following microorganisms, both in vitro and in clinical infections [see Indications and Usage (1)].
Gram-negative bacteria
Acinetobacter species
Bartonella bacilliformis
Brucella species
Campylobacter fetus
Enterobacter aerogenes
Escherichia coli
Francisella tularensis
Haemophilus ducreyi
Haemophilus influenzae
Klebsiella granulomatis
Klebsiella species
Neisseria gonorrhoeae
Shigella species
Vibrio cholerae
Yersinia pestis
Gram-positive bacteria
Bacillus anthracis
Listeria monocytogenes
Streptococcus pneumoniae
Anaerobic bacteria
Clostridium species
Fusobacterium fusiforme
Propionibacterium acnes
Other bacteria
Nocardiae and other aerobic Actinomyces species
Borrelia recurrentis
Chlamydophila psittaci
Chlamydia trachomatis
Mycoplasma pneumoniae
Rickettsiae species
Treponema pallidum
Treponema pallidum subspecies pertenue
Ureaplasma urealyticum
Parasites
Balantidium coli
Entamoeba species
Plasmodium falciparum*
*Doxycycline has been found to be active against the asexual erythrocytic forms of Plasmodium falciparum, but not against the gametocytes of P. falciparum. The precise mechanism of action of the drug is not known.
Susceptibility Testing Methods
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate carcinogenic potential of doxycycline hyclate have not been conducted.
However, a 2 year carcinogenicity study with doxycycline administered daily by oral gavage to adult rats (20, 75, 200 mg/kg/day) demonstrated an increase in uterine polyps in female rats at 200 mg/kg/day (10 times the maximum recommended daily adult dose of doxycycline hyclate based on body surface area comparison) with no change in tumor incidence in male rats at the same dose. A 2-year carcinogenicity study with doxycycline administered daily by oral gavage to adult male (maximum dose 150 mg/kg/day) and female (maximum dose 300 mg/kg/day) mice showed no changes in tumor incidence, at approximately 4 and 7 times the maximum recommended daily adult dose of doxycycline hyclate, based on a body surface area comparison, respectively.
Mutagenesis and fertility studies have not been conducted with doxycycline hyclate tablets. Mutagenesis studies with doxycycline demonstrated no potential to cause genetic toxicity in an in vitro point mutation study with mammalian cells or in an in vivo micronucleus assay in CD-1 mice. However, data from an in vitro mammalian chromosomal aberration assay conducted in CHO cells suggest that doxycycline is a weak clastogen. Oral administration of doxycycline to Sprague-Dawley rats showed adverse effects on fertility and reproduction including increased time for mating, reduced sperm motility, velocity and concentration as well as increased pre and post implantation loss. Reduced sperm velocity was seen at the lowest dosage tested, 50 mg/kg/day which is 2.5 times the maximum recommended daily adult dose of doxycycline hyclate tablets. Although doxycycline impairs the fertility of rats when administered at sufficient dosages, the effect of doxycycline hyclate tablets on human fertility is unknown.
13.2 Animal Toxicology and/or Pharmacology
Hyperpigmentation of the thyroid has been produced by members of the tetracycline-class in the following species: in rats by oxytetracycline, doxycycline, tetracycline PO4, and methacycline; in minipigs by doxycycline, minocycline, tetracycline PO4, and methacycline; in dogs by doxycycline and minocycline; in monkeys by minocycline.
Minocycline, tetracycline PO4, methacycline, doxycycline, tetracycline base, oxytetracycline HCl, and tetracycline HCl, were goitrogenic in rats fed a low iodine diet. This goitrogenic effect was accompanied by high radioactive iodine uptake. Administration of minocycline also produced a large goiter with high radioiodine uptake in rats fed a relatively high iodine diet.
Treatment of various animal species with this class of drugs has also resulted in the induction of thyroid hyperplasia in the following: in rats and dogs (minocycline); in chickens (chlortetracycline); and in rats and mice (oxytetracycline). Adrenal gland hyperplasia has been observed in goats and rats treated with oxytetracycline.
Results of animal studies indicate that tetracyclines cross the placenta and are found in fetal tissues.
-
15 REFERENCES
-
-
-
- 1. Friedman JM, Polifka JE. Teratogenic Effects of Drugs. A Resource for Clinicians (TERIS). Baltimore, MD: The Johns Hopkins University Press: 2000: 149-195.
- 2. Cziezel AE and Rockenbauer M. Teratogenic study of doxycycline. Obstet Gynecol 1997; 89: 524-528.
- 3. Horne HW Jr. and Kundsin RB. The role of mycoplasma among 81 consecutive pregnancies: a prospective study. Int J Fertil 1980; 25: 315-317.
- 4. Drugs and Lactation Database (LactMed) [Internet]. Bethesda (MD): National Library of Medicine (US); [Last Revision Date 2018 Oct 31; cited 2019 Jun]. Doxycycline; LactMed Record Number: 100; [about 3 screens]. Available from: http://toxnet.nlm.nih.gov/newtoxnet/lactmed.htm
-
-
-
-
16 HOW SUPPLIED/STORAGE AND HANDLING
How Supplied
Doxycycline hyclate tablets USP, 75 mg are round, blue, film-coated tablets, debossed with “PC90” on one side and blank on the other side. Each 75 mg tablet contains 86.6 mg of doxycycline hyclate equivalent to 75 mg of doxycycline.
Bottles of 60 tablets: NDC: 42806-645-60
Bottles of 100 tablets: NDC: 42806-645-01
Bottles of 500 tablets: NDC: 42806-645-05
Doxycycline hyclate tablets USP, 150 mg are capsule-shaped, green, film-coated tablets. Each side of the functionally scored tablet has two parallel score lines for splitting into 3 equal portions with “P” debossed on far-left, and “C” debossed on far-right portion of one side of the tablet, and “9” debossed on far-left and “1” debossed on far-right on the other side of the tablet. Each 150 mg tablet contains 173.2 mg of doxycycline hyclate equivalent to 150 mg of doxycycline.
Bottles of 60 tablets: NDC: 42806-646-60
Bottles of 100 tablets: NDC: 42806-646-01
Bottles of 500 tablets: NDC: 42806-646-05
Storage
Store at 20° to 25°C (68° to 77°F) excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature]. Protect from light and moisture. Dispense in a tight, light-resistant container as defined in the USP using a child-resistant closure.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Important Administration and Safety Information for Patients and Caregivers
Advise patients taking doxycycline hyclate tablets for malaria prophylaxis:
- that no present-day antimalarial agent, including doxycycline, guarantees protection against malaria.
- to avoid being bitten by mosquitoes by using personal protective measures that help avoid contact with mosquitoes, especially from dusk to dawn (for example, staying in well-screened areas, using mosquito nets, covering the body with clothing, and using an effective insect repellent).
- that doxycycline prophylaxis:
- - should begin 1 day to 2 days before travel to the malarious area,
- - should be continued daily while in the malarious area and after leaving the malarious area,
- - should be continued for 4 further weeks to avoid development of malaria after returning from an endemic area,
- - should not exceed 4 months.
Advise all patients taking doxycycline hyclate tablets:
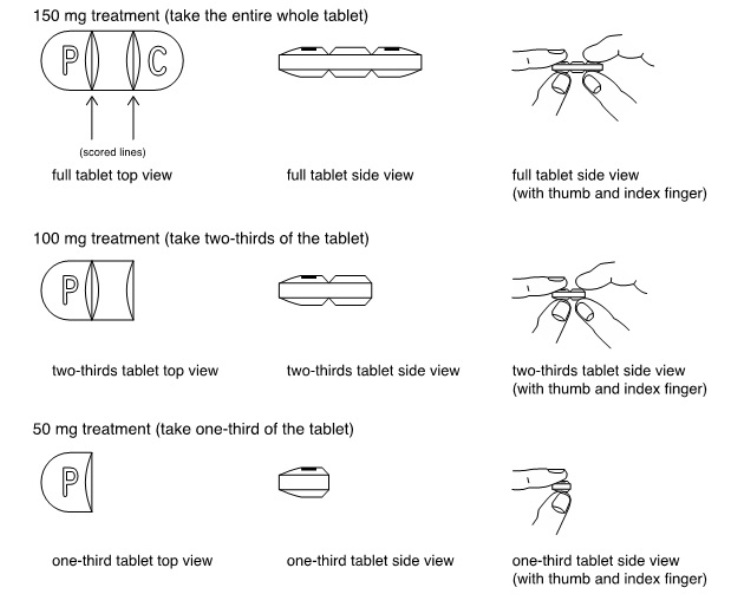
- that doxycycline hyclate tablets (150 mg) can be broken into two-thirds or one-third at the scored lines to provide 100 mg or 50 mg strength doses, respectively.
- that they must swallow doxycycline hyclate tablets whole. They must not break, open, crush, dissolve or chew the capsule [see Dosage and Administration (2.2)].
- to avoid excessive sunlight or artificial ultraviolet light while receiving doxycycline and to discontinue therapy if phototoxicity (for example, skin eruptions, etc.) occurs. Sunscreen or sunblock should be considered [see Warnings and Precautions (5.3)].
- to drink fluids liberally along with doxycycline hyclate tablets to reduce the risk of esophageal irritation and ulceration [see Adverse Reactions (6)].
- that the absorption of tetracyclines is reduced when taken with foods, especially those that contain calcium [see Drug Interactions (7.3)]. However, the absorption of doxycycline is not markedly influenced by simultaneous ingestion of food or milk [see Clinical Pharmacology (12.3)].
- that if gastric irritation occurs, doxycycline hyclate tablets may be given with food or milk [see Clinical Pharmacology (12.3)].
- that the absorption of tetracyclines is reduced when taken with antacids containing aluminum, calcium or magnesium, bismuth subsalicylate, and iron-containing preparations [see Drug Interactions (7.3)].
- that the use of doxycycline might increase the incidence of vaginal candidiasis.
Tooth Discoloration and Inhibition of Bone Growth
Advise patients that doxycycline hyclate tablets, like other tetracycline-class drugs, may cause permanent tooth discoloration of deciduous teeth and reversible inhibition of bone growth when administered during pregnancy. Tell your healthcare provider right away if you become pregnant during treatment [see Warnings and Precautions (5.1, 5.2) and Use in Specific Populations (8.1, 8.4)].
Lactation
Advise women not to breastfeed during treatment with doxycycline hyclate tablets and for 5 days after the last dose [see Use in Specific Populations (8.2)].
Diarrhea
Advise patients that diarrhea is a common problem caused by antibacterial drugs which usually ends when the antibacterial is discontinued. Sometimes after starting treatment with antibacterial drugs, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of antibacterial. If this occurs, patients should contact their physician as soon as possible.
Development of Resistance
Counsel patients that antibacterial drugs including doxycycline hyclate tablets should only be used to treat bacterial infections. They do not treat viral infections (for example, the common cold). When doxycycline hyclate tablets are prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by doxycycline hyclate tablets or other antibacterial drugs in the future.
All registered trademarks in this document are the property of their respective owners.
Distributed by:
Epic Pharma, LLC
Laurelton NY 11413
Rev. 05-2020-00
-
Instructions for Use
Doxycycline Hyclate
(DOX-i-SYE-kleen HYE-klate)
Tablets
for oral use
Rx Only
Read this Instructions for Use before you start using doxycycline hyclate tablets and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Note:
- Your healthcare provider may need to change your dose of doxycycline hyclate tablets during treatment as needed.
- Doxycycline hyclate tablets can be taken whole or broken at scored lines.
- Doxycycline hyclate tablets are marked with scored lines and may be broken at these scored lines to provide the following doses:
How to break your doxycycline hyclate tablets:
- Hold the tablet between your thumb and index finger close to the scored line for your dose of doxycycline hyclate tablets as shown above.
- Apply enough pressure to break the tablet at the scored line.
- Do not break the doxycycline hyclate tablets in any other way.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Distributed by:
Epic Pharma, LLC
Laurelton NY 11413
Rev. 05-2020-00
Rx only
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 75 mg 100ct
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL – 150 mg 60ct
-
INGREDIENTS AND APPEARANCE
DOXYCYCLINE HYCLATE
doxyclycline hyclate tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42806-645 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYCYCLINE HYCLATE (UNII: 19XTS3T51U) (DOXYCYCLINE ANHYDROUS - UNII:334895S862) DOXYCYCLINE ANHYDROUS 75 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color BLUE Score no score Shape ROUND Size 9mm Flavor Imprint Code PC90 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42806-645-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2020 2 NDC: 42806-645-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2020 3 NDC: 42806-645-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214207 12/16/2020 DOXYCYCLINE HYCLATE
doxyclycline hyclate tablet, coatedProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 42806-646 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOXYCYCLINE HYCLATE (UNII: 19XTS3T51U) (DOXYCYCLINE ANHYDROUS - UNII:334895S862) DOXYCYCLINE ANHYDROUS 150 mg Inactive Ingredients Ingredient Name Strength CARNAUBA WAX (UNII: R12CBM0EIZ) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SODIUM LAURYL SULFATE (UNII: 368GB5141J) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) WATER (UNII: 059QF0KO0R) Product Characteristics Color GREEN Score 3 pieces Shape OVAL (capsule-shaped) Size 16mm Flavor Imprint Code PC;91 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 42806-646-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2020 2 NDC: 42806-646-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2020 3 NDC: 42806-646-05 500 in 1 BOTTLE; Type 0: Not a Combination Product 12/16/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA214207 12/16/2020 Labeler - EPIC PHARMA, LLC (827915443) Registrant - EPIC PHARMA, LLC (827915443) Establishment Name Address ID/FEI Business Operations Caribe Holdings (Cayman) Co. Ltd. dba PuraCap Caribe 080230346 MANUFACTURE(42806-645, 42806-646)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.