ZODRYL AC 50- chlorpheniramine maleate and codeine phosphate suspension
Zodryl AC 50 by
Drug Labeling and Warnings
Zodryl AC 50 by is a Otc medication manufactured, distributed, or labeled by CodaDose, Inc., Gorbec Pharmaceutical Services Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
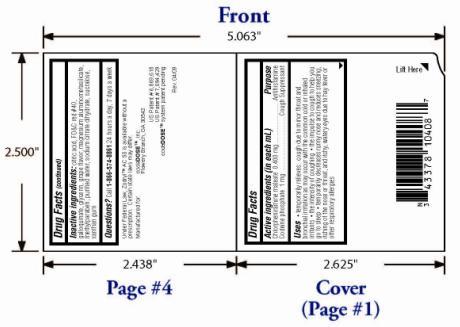
- OTC - ACTIVE INGREDIENT
-
OTC - PURPOSE
Temporarily relieves: cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants; the intensity of coughing; the impulse to cough to help you go to sleep; temporarily decreases runny nose and reduces sneezing, itching of the nose or throat, and itchy, watery eyes due to hay fever or other upper respiratory allergies
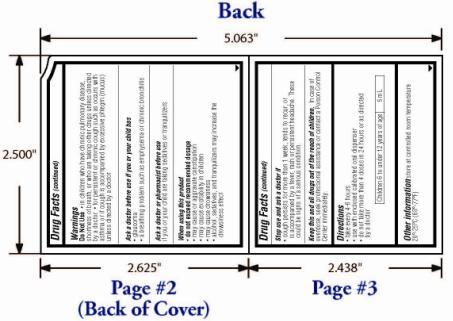
Warnings
- OTC - DO NOT USE
- OTC - ASK DOCTOR
- OTC - ASK DOCTOR/PHARMACIST
- OTC - WHEN USING
- OTC - STOP USE
-
OTC - KEEP OUT OF REACH OF CHILDREN
In case of overdose, seek professional assistance for contact a Poison Control Center immediately.
Directions:
-
Take every 4-6 hours
-
Use only with enclosed calibrated oral dispenser
-
Do not take more than 4 doses in 24 hours or as directed by a doctor
Children 6 to under 12 years of age: 5mL
Other information store at controlled room temperature 20°-25°C (68°-77°F).
INACTIVE INGREDIENT
Citric acid, FD&C red #40, galloquinate, glycerin, grape flavor, magnesium aluminometasilicate, methylparaben, purified water, sodium citrate dihydrate, sucralose, xanthan gum
-
- OTC - QUESTIONS
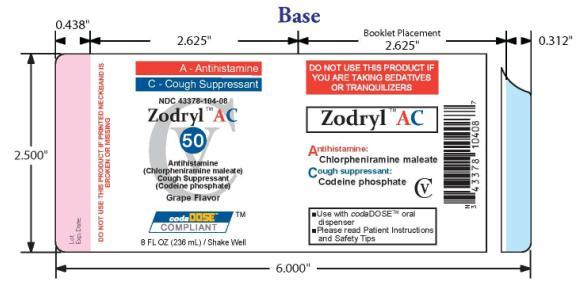
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ZODRYL AC 50
chlorpheniramine maleate and codeine phosphate suspensionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 43378-104 Route of Administration ORAL DEA Schedule CV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CHLORPHENIRAMINE MALEATE (UNII: V1Q0O9OJ9Z) (CHLORPHENIRAMINE - UNII:3U6IO1965U) CHLORPHENIRAMINE MALEATE 2 mg in 5 mL CODEINE PHOSPHATE (UNII: GSL05Y1MN6) (CODEINE - UNII:Q830PW7520) CODEINE PHOSPHATE 5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C RED NO. 40 (UNII: WZB9127XOA) TANNIC ACID (UNII: 28F9E0DJY6) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM ALUMINUM SILICATE (UNII: 6M3P64V0NC) METHYLPARABEN (UNII: A2I8C7HI9T) WATER (UNII: 059QF0KO0R) SODIUM CITRATE (UNII: 1Q73Q2JULR) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color red Score Shape Size Flavor GRAPE Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 43378-104-08 236 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 08/24/2009 Labeler - CodaDose, Inc. (831355115) Registrant - Gorbec Pharmaceutical Services Inc. (791919678) Establishment Name Address ID/FEI Business Operations Gorbec Pharmaceutical Services Inc. 791919678 manufacture
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.