Estazolam by Dr. Reddy's Labratories Inc. ESTAZOLAM tablet
Estazolam by
Drug Labeling and Warnings
Estazolam by is a Prescription medication manufactured, distributed, or labeled by Dr. Reddy's Labratories Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
-
BOXED WARNING
(What is this?)
WARNING: RISKS FROM CONCOMITANT USE WITH OPIOIDS; ABUSE, MISUSE, AND ADDICTION; and DEPENDENCE AND WITHDRAWAL REACTIONS
- Concomitant use of benzodiazepines and opioids may result in profound sedation, respiratory depression, coma, and death (see WARNINGS).
- Reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients for signs and symptoms of respiratory depression and sedation. (see WARNINGS and PRECAUTIONS).
- The use of benzodiazepines, including Estazolam, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes. Before prescribing Estazolam and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (see WARNINGS).
- The continued use of benzodiazepines, including Estazolam, may lead to clinically significant physical dependence. The risks of dependence and withdrawal increase with longer treatment duration and higher daily dose. Abrupt discontinuation or rapid dosage reduction of Estazolam after continued use may precipitate acute withdrawal reactions, which can be life-threatening. To reduce the risk of withdrawal reactions, use a gradual taper to discontinue Estazolam or reduce the dosage (see DOSAGE AND ADMINISTRATION and WARNINGS).
-
DESCRIPTION
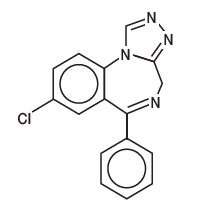
Estazolam, a triazolobenzodiazepine derivative, is an oral hypnotic agent. Estazolam occurs as a fine, white, odorless powder that is soluble in alcohol and practically insoluble in water. The chemical name for estazolam is 8-chloro-6-phenyl-4H-s-triazolo[4,3-α][1,4]benzodiazepine. The structural formula is represented as follows:
C16H11ClN4
Each tablet, for oral administration, contains either 1 mg or 2 mg of estazolam, USP. In addition, each tablet contains the following inactive ingredients: 1 mg tablets - corn starch, lactose monohydrate, pregelatinized starch, and stearic acid; 2 mg tablets - corn starch, FD&C Red #40 aluminum lake, FD&C Yellow #6 aluminum lake, lactose monohydrate, pregelatinized starch, and stearic acid.
-
CLINICAL PHARMACOLOGY
Pharmacokinetics
Absorption
Estazolam tablets have been found to be equivalent in absorption to an orally administered solution of estazolam. In healthy subjects who received up to three times the recommended dose of estazolam, peak estazolam plasma concentrations occurred within two hours after dosing (range 0.5 to 6.0 hours) and were proportional to the administered dose, suggesting linear pharmacokinetics over the dosage range tested.
Metabolism
Estazolam is extensively metabolized. Only two metabolites (1-oxo-estazolam & 4-hydroxy-estazolam) were detected in human plasma up to 18 hrs.
The pharmacologic activity of estazolam is primarily from the parent drug. The elimination of the parent drug takes place via hepatic metabolism of estazolam to hydroxylated and other metabolites that are eliminated largely in the urine both free and conjugated. In humans, greater than 70% of a single dose of estazolam was recovered in the urine as metabolites. Less than 5% of a 2 mg dose of estazolam was excreted unchanged in the urine, with only 4% of the dose appearing in the feces. The principal urinary excretion product is an unidentified metabolite, presumed to be a metabolic product of 4-hydroxy- estazolam, accounting for at least 27% of the administered dose. 4-hydroxy-estazolam is the major metabolite in plasma, with concentrations approaching 12% of those of the parent eight hours after administration. Urinary 4-hydroxy-estazolam and 1-oxo-estazolam account for 11.9% and 4.4% of the dose respectively. In vitro studies with human liver microsomes indicate that the biotransformation of estazolam to the major circulating metabolite 4-hydroxy-estazolam is mediated by cytochrome P450 3A (CYP3A). While 4-hydroxy-estazolam and the lesser metabolite, 1-oxo-estazolam, have some pharmacologic activity, their low potencies and low concentrations preclude any significant contribution to the hypnotic effect of estazolam.
Elimination
The range of estimates for the mean elimination half-life of estazolam varied from 10 to 24 hours. Radiolabel mass balance studies indicate that the main route of excretion is via the kidneys. After 5 days, 87% of the administered radioactivity was excreted in human urine. Less than 4% of the dose was excreted unchanged. Eleven metabolites were found in urine. Four metabolites were identified as 1-oxo-estazolam, 4'-hydroxy-estazolam, 4-hydroxy-estazolam, and benzophenone, as free metabolites and glucuronides. The predominant metabolite in urine (17% of the administered dose) has not been identified, but is likely to be a metabolite of 4-hydroxy-estazolam.
Special Populations
In a small study (N = 8) using various doses in older subjects (59 to 68 years), peak estazolam concentrations were found to be similar to those observed in younger subjects with a mean elimination half-life of 18.4 hours (range 13.5 to 34.6 hours). The influence of hepatic or renal impairment on the pharmacokinetics of estazolam has not been studied.
Cigarette smoking
The clearance of benzodiazepines is accelerated in smokers compared to nonsmokers, and there is evidence that this occurs with estazolam. This decrease in half-life, presumably due to enzyme induction by smoking, is consistent with other drugs with similar hepatic clearance characteristics. In all subjects and at all doses, the mean elimination half-life appeared to be independent of the dose.
Drug-drug interaction
The metabolism of estazolam to the major circulating metabolite 4-hydroxy-estazolam is catalyzed by CYP3A. While no in vivo drug-drug interaction studies were conducted between estazolam and inhibitors/inducers of CYP3A, compounds that are potent CYP3A inhibitors (such as ketoconazole, itraconazole, nefazodone, fluvoxamine, and erythromycin) would be expected to increase plasma estazolam concentrations and CYP3A inducers (such as carbamazepine, phenytoin, rifampin and barbiturates) would be expected to decrease estazolam concentrations.
Drug interaction with fluoxetine
A multiple-dose study was conducted to assess the effect of fluoxetine 20 mg BID on the pharmacokinetics of estazolam 2 mg QHS after seven days. The pharmacokinetics of estazolam (Cmax and AUC) were not affected during multiple-dose fluoxetine, suggesting no clinically significant pharmacokinetic interaction.
The ability of estazolam to induce or inhibit human enzyme systems
The results from in vitro human liver microsomal studies suggest that at therapeutic concentrations, estazolam has no significant inhibitory effect on the major human cytochrome P450 enzyme activities (i.e., CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A). The ability of estazolam to induce human hepatic enzyme systems has not been determined.
Pharmacodynamics
Postulated relationship between elimination rate of benzodiazepine hypnotics and their profile of common untoward effects
The type and duration of hypnotic effects and the profile of unwanted effects during administration of benzodiazepine drugs may be influenced by the biologic half-life of administered drug and any active metabolites formed. If half-lives are long, drug or metabolites may accumulate during periods of nightly administration and may be associated with impairments of cognitive and/or motor performance during waking hours; the possibility of interaction with other psychoactive drugs or alcohol will be increased. In contrast, if half-lives are short, drug and metabolites will be cleared before the next dose is ingested, and carry-over effects related to excessive sedation or CNS depression should be minimal or absent. However, during nightly use for an extended period, pharmacodynamic tolerance or adaptation to some effects of benzodiazepine hypnotics may develop. If the drug has a short elimination half-life, it is possible that a relative deficiency of the drug or its active metabolites (i.e., in relationship to the receptor site) may occur at some point in the interval between each night's use. This sequence of events may account for two clinical findings reported to occur after several weeks of max nightly use of rapidly eliminated benzodiazepine hypnotics, namely, increased wakefulness during the last third of the night and increased daytime anxiety in selected patients.
-
CLINICAL STUDIES
Controlled Trials Supporting Efficacy
In three 7 night, double-blind, parallel-group trials comparing estazolam 1 mg and/or 2 mg with placebo in adult outpatients with chronic insomnia, estazolam 2 mg was consistently superior to placebo in subjective measures of sleep induction (latency) and sleep maintenance (duration, number of awakenings, depth and quality of sleep); estazolam 1 mg was similarly superior to placebo on all measures of sleep maintenance, however, it significantly improved sleep induction in only one of two studies. In a similarly designed trial comparing estazolam 0.5 mg and 1 mg with placebo in geriatric outpatients with chronic insomnia, only the 1 mg estazolam dose was consistently superior to placebo in sleep induction (latency) and in only one measure of sleep maintenance (i.e., duration of sleep).
In a single-night, double-blind, parallel-group trial comparing estazolam 2 mg and placebo in patients admitted for elective surgery and requiring sleep medications, estazolam was superior to placebo in subjective measures of sleep induction and maintenance.
In a 12 week, double-blind, parallel-group trial including a comparison of estazolam 2 mg and placebo in adult outpatients with chronic insomnia, estazolam was superior to placebo in subjective measures of sleep induction (latency) and maintenance (duration, number of awakenings, total wake time during sleep) at week 2, but produced consistent improvement over 12 weeks only for sleep duration and total wake time during sleep. Following withdrawal at week 12, rebound insomnia was seen at the first withdrawal week, but there was no difference between drug and placebo by the second withdrawal week in all parameters except latency, for which normalization did not occur until the fourth withdrawal week.
Adult outpatients with chronic insomnia were evaluated in a sleep laboratory trial comparing four doses of estazolam (0.25, 0.5, 1 and 2 mg) and placebo, each administered for 2 nights in a crossover design. The higher estazolam doses were superior to placebo in most EEG measures of sleep induction and maintenance, especially at the 2 mg dose, but only for sleep duration in subjective measures of sleep.
-
INDICATIONS AND USAGE
Estazolam is indicated for the short-term management of insomnia characterized by difficulty in falling asleep, frequent nocturnal awakenings, and/or early morning awakenings. Both outpatient studies and a sleep laboratory study have shown that estazolam administered at bedtime improved sleep induction and sleep maintenance (see CLINICAL PHARMACOLOGY).
Because insomnia is often transient and intermittent, the prolonged administration of estazolam is generally neither necessary nor recommended. Since insomnia may be a symptom of several other disorders, the possibility that the complaint may be related to a condition for which there is a more specific treatment should be considered.
There is evidence to support the ability of estazolam to enhance the duration and quality of sleep for intervals up to 12 weeks (see CLINICAL PHARMACOLOGY).
-
CONTRAINDICATIONS
Estazolam is contraindicated with ketoconazole and itraconazole, since these medications significantly impair oxidative metabolism mediated by CYP3A (see WARNINGS and PRECAUTIONS, Drug Interactions).
-
WARNINGS
Risks from Concomitant Use with Opioids
Concomitant use of benzodiazepines, including estazolam, and opioids may result in profound sedation, respiratory depression, coma, and death. Because of these risks, reserve concomitant prescribing of these drugs in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioids alone. If a decision is made to prescribe estazolam concomitantly with opioids, prescribe the lowest effective dosages and minimum durations of concomitant use, and follow patients closely for signs and symptoms of respiratory depression and sedation. In patients already receiving an opioid analgesic, prescribe a lower initial dose of estazolam than indicated in the absence of an opioid and titrate based on clinical response. If an opioid is initiated in a patient already taking estazolam, prescribe a lower initial dose of the opioid and titrate based upon clinical response.
Advise both patients and caregivers about the risks of respiratory depression and sedation when estazolam is used with opioids. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see PRECAUTIONS, Drug Interactions).
Abuse, Misuse, and Addiction
The use of benzodiazepines, including estazolam, exposes users to the risks of abuse, misuse, and addiction, which can lead to overdose or death. Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death (see DRUG ABUSE AND DEPENDENCE, Abuse).
Before prescribing estazolam and throughout treatment, assess each patient's risk for abuse, misuse, and addiction (e.g., using a standardized screening tool). Use of estazolam, particularly in patients at elevated risk, necessitates counseling about the risks and proper use of estazolam along with monitoring for signs and symptoms of abuse, misuse, and addiction. Prescribe the lowest effective dosage; avoid or minimize concomitant use of CNS depressants and other substances associated with abuse, misuse, and addiction (e.g., opioid analgesics, stimulants); and advise patients on the proper disposal of unused drug. If a substance use disorder is suspected, evaluate the patient and institute (or refer them for) early treatment, as appropriate.
Dependence and Withdrawal Reactions
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue estazolam or reduce the dosage (a patient-specific plan should be used to taper the dose) (see DOSAGE AND ADMINISTRATION, Discontinuation or Dosage Reduction of Estazolam).
Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages, and those who have had longer durations of use.
Acute Withdrawal Reactions
The continued use of benzodiazepines, including estazolam, may lead to clinically significant physical dependence. Abrupt discontinuation or rapid dosage reduction of estazolam after continued use, or administration of flumazenil (a benzodiazepine antagonist) may precipitate acute withdrawal reactions, which can be life-threatening (e.g., seizures) (see DRUG ABUSE AND DEPENDENCE, Dependence).
Protracted Withdrawal Syndrome
In some cases, benzodiazepine users have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months (see DRUG ABUSE AND DEPENDENCE, Dependence).
Because sleep disturbances may be the presenting manifestation of a physical and/or psychiatric disorder, symptomatic treatment of insomnia should be initiated only after a careful evaluation of the patient. The failure of insomnia to remit after 7 to 10 days of treatment may indicate the presence of a primary psychiatric and/or medical illness that should be evaluated. Worsening of insomnia or the emergence of new thinking or behavior abnormalities may be the consequence of an unrecognized psychiatric or physical disorder. Such findings have emerged during the course of treatment with sedative-hypnotic drugs. Because some of the important adverse effects of sedative-hypnotics appear to be dose-related (see PRECAUTIONS and DOSAGE AND ADMINISTRATION), it is important to use the smallest possible effective dose, especially in the elderly.
Complex behaviors such as "sleep-driving" (i.e., driving while not fully awake after ingestion of a sedative-hypnotic, with amnesia for the event) have been reported. These events can occur in sedative- hypnotic-naïve as well as in sedative-hypnotic-experienced persons. Although behaviors such as sleep-driving may occur with sedative-hypnotics alone at therapeutic doses, the use of alcohol and other CNS depressants with sedative-hypnotics appears to increase the risk of such behaviors, as does the use of sedative-hypnotics at doses exceeding the maximum recommended dose. Due to the risk to the patient and the community, discontinuation of sedative-hypnotics should be strongly considered for patients who report a "sleep-driving" episode.
Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Because sedative-hypnotics can cause drowsiness and a decreased level of consciousness, patients, particularly the elderly, are at higher risk of falls.
Severe Anaphylactic and Anaphylactoid Reactions
Rare cases of angioedema involving the tongue, glottis or larynx have been reported in patients after taking the first or subsequent doses of sedative-hypnotics, including estazolam. Some patients have had additional symptoms such as dyspnea, throat closing, or nausea and vomiting that suggest anaphylaxis. Some patients have required medical therapy in the emergency department. If angioedema involves the tongue, glottis or larynx, airway obstruction may occur and be fatal. Patients who develop angioedema after treatment with estazolam should not be rechallenged with the drug.
Estazolam, like other benzodiazepines, has CNS depressant effects. For this reason, patients should be cautioned against engaging in hazardous occupations requiring complete mental alertness, such as operating machinery or driving a motor vehicle, after ingesting the drug, including potential impairment of the performance of such activities that may occur the day following ingestion of estazolam. Patients should also be cautioned about possible combined effects with alcohol and other CNS depressant drugs.
As with all benzodiazepines, amnesia, paradoxical reactions (e.g., excitement, agitation, etc.), and other adverse behavioral effects may occur unpredictably.
Estazolam Interaction with Drugs That Inhibit Metabolism via Cytochrome P450 3A (CYP3A)
The metabolism of estazolam to the major circulating metabolite 4-hydroxy-estazolam and the metabolism of other triazolobenzodiazepines is catalyzed by CYP3A. Consequently, estazolam should be avoided in patients receiving ketoconazole and itraconazole, which are very potent inhibitors of CYP3A (see CONTRAINDICATIONS). With drugs inhibiting CYP3A to a lesser, but still significant degree, estazolam should be used only with caution and consideration of appropriate dosage reduction. The following are examples of drugs known to inhibit the metabolism of other related benzodiazepines, presumably through inhibition of CYP3A: nefazodone, fluvoxamine, cimetidine, diltiazem, isoniazide, and some macrolide antibiotics.
While no in vivo drug-drug interaction studies were conducted between estazolam and inducers of CYP3A, compounds that are potent CYP3A inducers (such as carbamazepine, phenytoin, rifampin, and barbiturates) would be expected to decrease estazolam concentrations.
Neonatal Sedation and Withdrawal Syndrome
Use of estazolam late in pregnancy can result in sedation (respiratory depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in the neonate (see PRECAUTIONS: Pregnancy). Monitor neonates exposed to estazolam during pregnancy or labor for signs of sedation and monitor neonates exposed to estazolam during pregnancy for signs of withdrawal; manage these neonates accordingly.
-
PRECAUTIONS
General
Impaired motor and/or cognitive performance attributable to the accumulation of benzodiazepines and their active metabolites following several days of repeated use at their recommended doses is a concern in certain vulnerable patients (e.g., those especially sensitive to the effects of benzodiazepines or those with a reduced capacity to metabolize and eliminate them) (see DOSAGE AND ADMINISTRATION).
Elderly or debilitated patients and those with impaired renal or hepatic function should be cautioned about these risks and advised to monitor themselves for signs of excessive sedation or impaired conditions.
Estazolam appears to cause dose-related respiratory depression that is ordinarily not clinically relevant at recommended doses in patients with normal respiratory function. However, patients with compromised respiratory function may be at risk and should be monitored appropriately. As a class, benzodiazepines have the capacity to depress respiratory drive; there are insufficient data available, however, to characterize their relative potency in depressing respiratory drive at clinically recommended doses.
As with other benzodiazepines, estazolam should be administered with caution to patients exhibiting signs or symptoms of depression. Suicidal tendencies may be present in such patients and protective measures may be required. Intentional overdosage is more common in this group of patients; therefore, the least amount of drug that is feasible should be prescribed for the patient at any one time.
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Risks from Concomitant Use with Opioids
Advise both patients and caregivers about the risks of potentially fatal respiratory depression and sedation when estazolam is used with opioids and not to use such drugs concomitantly unless supervised by a health care provider. Advise patients not to drive or operate heavy machinery until the effects of concomitant use with the opioid have been determined (see WARNINGS: Risks from Concomitant Use with Opioids and PRECAUTIONS, Drug Interactions).
Abuse, Misuse, and Addiction
Inform patients that the use of estazolam, even at recommended dosages, exposes users to risks of abuse, misuse, and addiction, which can lead to overdose and death, especially when used in combination with other medications (e.g., opioid analgesics), alcohol, and/or illicit substances. Inform patients about the signs and symptoms of benzodiazepine abuse, misuse, and addiction; to seek medical help if they develop these signs and/or symptoms; and on the proper disposal of unused drug (see WARNINGS, Abuse, Misuse, and Addiction and DRUG ABUSE AND DEPENDENCE).
Withdrawal Reactions
Inform patients that the continued use of estazolam may lead to clinically significant physical dependence and that abrupt discontinuation or rapid dosage reduction of estazolam may precipitate acute withdrawal reactions, which can be life-threatening. Inform patients that in some cases, patients taking benzodiazepines have developed a protracted withdrawal syndrome with withdrawal symptoms lasting weeks to more than 12 months. Instruct patients that discontinuation or dosage reduction of estazolam may require a slow taper (see WARNINGS, Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE).
"Sleep-Driving" and Other Complex Behaviors
There have been reports of people getting out of bed after taking a sedative-hypnotic and driving their cars while not fully awake, often with no memory of the event. If a patient experiences such an episode, it should be reported to his or her doctor immediately, since "sleep-driving" can be dangerous. This behavior is more likely to occur when sedative-hypnotics are taken with alcohol or other central nervous system depressants (see WARNINGS). Other complex behaviors (e.g., preparing and eating food, making phone calls, or having sex) have been reported in patients who are not fully awake after taking a sedative-hypnotic. As with sleep-driving, patients usually do not remember these events.
Inform your physician about any alcohol consumption and medicine you are taking now, including drugs you may buy without a prescription. Alcohol should not be used during treatment with hypnotics.
Inform your physician if you are planning to become pregnant, if you are pregnant, or if you become pregnant while you are taking this medicine.
You should not take this medicine if you are nursing, as the drug may be excreted in breast milk.
Until you experience the way this medicine affects you, do not drive a car, operate potentially dangerous machinery, or engage in hazardous occupations requiring complete mental alertness after taking this medicine.
Advise patients that increased drowsiness and decreased consciousness may increase the risk of falls in some patients.
Pregnancy
Advise pregnant females that use of estazolam late in pregnancy can results in sedation (respiratory, depression, lethargy, hypotonia) and/or withdrawal symptoms (hyperreflexia, irritability, restlessness, tremors, inconsolable crying, and feeding difficulties) in newborns (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS: Pregnancy). Instruct patients to inform their healthcare provider if they are pregnant.
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to estazolam during pregnancy (see Precautions, Pregnancy).
Nursing
Advise patients that breastfeeding is not recommended during treatment with estazolam (see PRECAUTIONS: Nursing Mothers).
Laboratory Tests
Laboratory tests are not ordinarily required in otherwise healthy patients. When treatment with estazolam is protracted, periodic blood counts, urinalyses, and blood chemistry analyses are advisable.
Drug Interactions
The concomitant use of benzodiazepines and opioids increases the risk of respiratory depression because of actions at different receptor sites in the CNS that control respiration. Benzodiazepines interact at GABAA sites and opioids interact primarily at mu receptors. When benzodiazepines and opioids are combined, the potential for benzodiazepines to significantly worsen opioid-related respiratory depression exists.
Limit dosage and duration of concomitant use of benzodiazepines and opioids, and monitor patients closely for respiratory depression and sedation.
If estazolam is given concomitantly with other drugs acting on the central nervous system, careful consideration should be given to the pharmacology of all agents. The action of the benzodiazepines may be potentiated by anticonvulsants, antihistamines, alcohol, barbiturates, monoamine oxidase inhibitors, narcotics, phenothiazines, psychotropic medications, or other drugs that produce CNS depression. Smokers have an increased clearance of benzodiazepines as compared to nonsmokers; this was seen in studies with estazolam (see CLINICAL PHARMACOLOGY).
While no in vivo drug-drug interaction studies were conducted between estazolam and inducers of CYP3A, compounds that are potent CYP3A inducers (such as carbamazepine, phenytoin, rifampin, and barbiturates) would be expected to decrease estazolam concentrations.
Estazolam Interaction with Drugs That Inhibit Metabolism via Cytochrome P450 3A (CYP3A)
The metabolism of estazolam to the major circulating metabolite 4-hydroxy-estazolam and the metabolism of other triazolobenzodiazepines is catalyzed by CYP3A. Consequently, estazolam should be avoided in patients receiving ketoconazole and itraconazole, which are very potent inhibitors of CYP3A (see CONTRAINDICATIONS). With drugs inhibiting CYP3A to a lesser, but still significant degree, estazolam should be used only with caution and consideration of appropriate dosage reduction. The following are examples of drugs known to inhibit the metabolism of other related benzodiazepines, presumably through inhibition of CYP3A: nefazodone, fluvoxamine, cimetidine, diltiazem, isoniazide, and some macrolide antibiotics.
Drug interaction with fluoxetine
A multiple-dose study was conducted to assess the effect of fluoxetine 20 mg BID on the pharmacokinetics of estazolam 2 mg QHS after seven days. The pharmacokinetics of estazolam (Cmax and AUC) were not affected during multiple-dose fluoxetine, suggesting no clinically significant pharmacokinetic interaction.
Estazolam Interaction with Other Drugs That are Metabolized by Cytochrome P450 (CYP)
At clinically relevant concentrations, in vitro studies indicate that estazolam (0.6µM) was not inhibitory towards the major cytochrome P450 isoforms CYP1A2, CYP2A6, CYP2C9, CYP2C19, CYP2D6, CYP2E1, and CYP3A. Therefore, based on these in vitro data, estazolam is very unlikely to inhibit the biotransformation of other drugs metabolized by these CYP isoforms.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Two-year carcinogenicity studies were conducted in mice and rats at dietary doses of 0.8, 3, and 10 mg/kg/day and 0.5, 2, and 10 mg/kg/day, respectively. Evidence of tumorigenicity was not observed in either study. Incidence of hyperplastic liver nodules increased in female mice given the mid- and high- dose levels. The significance of such nodules in mice is not known at this time.
In vitro and in vivo mutagenicity tests including the Ames test, DNA repair in B. subtilis, in vivo cytogenetics in mice and rats, and the dominant lethal test in mice did not show a mutagenic potential for estazolam.
Fertility in male and female rats was not affected by doses up to 30 times the usual recommended human dose.
Pregnancy
Pregnancy Exposure Registry
There is a pregnancy registry that monitors pregnancy outcomes in women exposed to psychiatric medications, including estazolam, during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Psychiatric Medications at 1-866-961-2388 or visiting online at https://womensmentalhealth.org/pregnancyregistry/.
Risk Summary
Neonates born to mothers using benzodiazepines late in pregnancy have been reported to experience symptoms of sedation and/or neonatal withdrawal (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome and PRECAUTIONS: Clinical Considerations). Available data from published observational studies of pregnant women exposed to benzodiazepines do not report a clear association with benzodiazepines and major birth defects (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated risk of major birth defects and of miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Benzodiazepines cross the placenta and may produce respiratory depression, hypotonia and sedation in neonates. Monitor neonates exposed to estazolam during pregnancy and labor for signs of sedation, respiratory depression, hypotonia, and feed problems. Monitor neonates exposed to estazolam during pregnancy for signs of withdrawal. Manage these neonates accordingly (see WARNINGS: Neonatal Sedation and Withdrawal Syndrome).
Data
Human Data
Published data from observational studies on the use of benzodiazepines during pregnancy do not report a clear association with benzodiazepines and major birth defects. Although early studies reported an increased risk of congenital malformations with diazepam and chlordiazepoxide, there was no consistent pattern noted. In addition, the majority of more recent case-control and cohort studies of benzodiazepine use during pregnancy, which were adjusted for confounding exposures to alcohol, tobacco and other medications, have not confirmed these findings.
Nursing Mothers
There are no data on the presence of estazolam in human milk. Estazolam is present in animal milk. When a drug is present in animal milk, it is likely that the drug will be present in human milk. There are reports that sedation, poor feeding and poor weight gain in infants exposed to benzodiazepines through breast milk. The effects of estazolam on milk production are unknown. Because of estazolam's long half-life, the potential for estazolam to accumulate in breast milk, and the potential for serious adverse reactions, including sedation and withdrawal symptoms in breastfed infants, advise patients that breastfeeding is not recommended during treatment with estazolam.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 18 have not been established.
Geriatric Use
Approximately 18% of individuals participating in the premarketing clinical trials of estazolam were 60 years of age or older. Overall, the adverse event profile did not differ substantively from that observed in younger individuals. Care should be exercised when prescribing benzodiazepines to small or debilitated elderly patients (see DOSAGE AND ADMINISTRATION).
-
ADVERSE REACTIONS
Commonly Observed
The most commonly observed adverse events associated with the use of estazolam, not seen at an equivalent incidence among placebo-treated patients were somnolence, hypokinesia, dizziness, and abnormal coordination.
Associated with Discontinuation of Treatment
Approximately 3% of 1277 patients who received estazolam in U.S. premarketing clinical trials discontinued treatment because of an adverse clinical event. The only event commonly associated with discontinuation, accounting for 1.3% of the total, was somnolence.
Incidence in Controlled Clinical Trials
The table below enumerates adverse events that occurred at an incidence of 1% or greater among patients with insomnia who received estazolam in 7-night, placebo-controlled trials. Events reported by investigators were classified into standard dictionary (COSTART) terms to establish event frequencies. Event frequencies reported were not corrected for the occurrence of these events at baseline. The frequencies were obtained from data pooled across six studies: estazolam, N = 685; placebo, N = 433. The prescriber should be aware that these figures cannot be used to predict the incidence of side effects in the course of usual medical practice in which patient characteristics and other factors differ from those that prevailed in these six clinical trials. Similarly, the cited frequencies cannot be compared with figures obtained from other clinical investigators involving related drug products and uses, since each group of drug trials was conducted under a different set of conditions. However, the cited figures provide the physician with a basis of estimating the relative contribution of drug and nondrug factors to the incidence of side effects in the population studied.
INCIDENCE OF ADVERSE EXPERIENCES IN PLACEBO-CONTROLLED CLINICAL TRIALS (Percentage of Patients Reporting) Body System/Adverse Event* Estazolam (N = 685) Placebo (N = 433) - * Events reported by at least 1% of estazolam patients.
Body as a Whole Headache 16 27 Asthenia 11 8 Malaise 5 5 Lower extremity pain 3 2 Back pain 2 2 Body pain 2 2 Abdominal pain 1 2 Chest pain 1 1 Digestive System Nausea 4 5 Dyspepsia 2 2 Musculoskeletal System Stiffness 1 - Nervous System Somnolence 42 27 Hypokinesia 8 4 Nervousness 8 11 Dizziness 7 3 Coordination abnormal 4 1 Hangover 3 2 Confusion 2 - Depression 2 3 Dream abnormal 2 2 Thinking abnormal 2 1 Respiratory System Cold symptoms 3 5 Pharyngitis 1 2 Skin and Appendages Pruritus 1 - Other Adverse Events
During clinical trials, some of which were not placebo-controlled, estazolam was administered to approximately 1300 patients. Untoward events associated with this exposure were recorded by clinical investigators using terminology of their own choosing. To provide a meaningful estimate of the proportion of individuals experiencing adverse events, similar types of untoward events must be grouped into a smaller number of standardized event categories. In the tabulations that follow, a standard COSTART dictionary terminology has been used to classify reported adverse events. The frequencies presented, therefore, represent the proportion of the 1277 individuals exposed to estazolam who experienced an event of the type cited on at least one occasion while receiving estazolam. All reported events are included except those already listed in the previous table, those COSTART terms too general to be informative, and those events where a drug cause was remote. Events are further classified within body system categories and enumerated in order of decreasing frequency using the following definitions: frequent adverse events are defined as those occurring on one or more occasions in at least 1/100 patients; infrequent adverse events are those occurring in 1/100 to 1/1000 patients; rare events are those occurring in less than 1/1000 patients. It is important to emphasize that, although the events reported did occur during treatment with estazolam, they were not necessarily caused by it.
Body as a Whole - Infrequent: allergic reaction, chills, fever, neck pain, upper extremity pain; Rare: edema, jaw pain, swollen breast.
Cardiovascular System - Infrequent: flushing, palpitation; Rare: arrhythmia, syncope.
Digestive System - Frequent: constipation, dry mouth; Infrequent: decreased appetite, flatulence, gastritis, increased appetite, vomiting; Rare: enterocolitis, melena, ulceration of the mouth.
Endocrine System - Rare: thyroid nodule.
Hematologic and Lymphatic System - Rare: leukopenia, purpura, swollen lymph nodes.
Metabolic/Nutritional Disorders - Infrequent: thirst; Rare: increased SGOT, weight gain, weight loss.
Musculoskeletal System - Infrequent: arthritis, muscle spasm, myalgia; Rare: arthralgia.
Nervous System - Frequent: anxiety; Infrequent: agitation, amnesia, apathy, emotional lability, euphoria, hostility, paresthesia, seizure, sleep disorder, stupor, twitch; Rare: ataxia, circumoral paresthesia, decreased libido, decreased reflexes, hallucinations, neuritis, nystagmus, tremor.
Minor changes in EEG patterns, usually low-voltage fast activity, have been observed in patients during estazolam therapy or withdrawal and are of no known clinical significance.
Respiratory System - Infrequent: asthma, cough, dyspnea, rhinitis, sinusitis; Rare: epistaxis, hyperventilation, laryngitis.
Skin and Appendages - Infrequent: rash, sweating, urticaria; Rare: acne, dry skin.
Special Senses - Infrequent: abnormal vision, ear pain, eye irritation, eye pain, eye swelling, perverse taste, photophobia, tinnitus; Rare: decreased hearing, diplopia, scotomata.
Urogenital System - Infrequent: frequent urination, menstrual cramps, urinary hesitancy, urinary urgency, vaginal discharge/itching; Rare: hematuria, nocturia, oliguria, penile discharge, urinary incontinence.
Postintroduction Reports - Voluntary reports of non-U.S. postmarketing experience with estazolam have included rare occurrences of photosensitivity, Stevens-Johnson syndrome, and agranulocytosis.
Because of the uncontrolled nature of these spontaneous reports, a causal relationship to estazolam treatment has not been determined.
To report SUSPECTED ADVERSE EVENTS, contact Dr.Reddy’s Laboratories Inc., at 1-888-375-3784 or FDA at 1-800-FDA-1088 or http://www.fda.gov/medwatch for voluntary reporting of adverse reactions.
-
DRUG ABUSE AND DEPENDENCE
Abuse
Estazolam is a benzodiazepine and a CNS depressant with a potential for abuse and addiction. Abuse is the intentional, non-therapeutic use of a drug, even once, for its desirable psychological or physiological effects. Misuse is the intentional use, for therapeutic purposes, of a drug by an individual in a way other than prescribed by a health care provider or for whom it was not prescribed. Drug addiction is a cluster of behavioral, cognitive, and physiological phenomena that may include a strong desire to take the drug, difficulties in controlling drug use (e.g., continuing drug use despite harmful consequences, giving a higher priority to drug use than other activities and obligations), and possible tolerance or physical dependence. Even taking benzodiazepines as prescribed may put patients at risk for abuse and misuse of their medication. Abuse and misuse may lead to addiction.
Abuse and misuse of benzodiazepines often (but not always) involve the use of doses greater than the maximum recommended dosage and commonly involve concomitant use of other medications, alcohol, and/or illicit substances, which is associated with an increased frequency of serious adverse outcomes, including respiratory depression, overdose, or death. Benzodiazepines are often sought by individuals who abuse drugs and other substances, and by individuals with addictive disorders (see WARNINGS, Abuse, Misuse, and Addiction).
The following adverse reactions have occurred with benzodiazepine abuse and/or misuse: abdominal pain, amnesia, anorexia, anxiety, aggression, ataxia, blurred vision, confusion, depression, disinhibition, disorientation, dizziness, euphoria, impaired concentration and memory, indigestion, irritability, muscle pain, slurred speech, tremors, and vertigo.
The following severe adverse reactions have occurred with benzodiazepine abuse and/or misuse: delirium, paranoia, suicidal ideation and behavior, seizures, coma, breathing difficulty, and death. Death is more often associated with polysubstance use (especially benzodiazepines with other CNS depressants such as opioids and alcohol).
Dependence
Physical Dependence
Estazolam may produce physical dependence from continued therapy. Physical dependence is a state that develops as a result of physiological adaptation in response to repeated drug use, manifested by withdrawal signs and symptoms after abrupt discontinuation or a significant dose reduction of a drug. Abrupt discontinuation or rapid dosage reduction of benzodiazepines or administration of flumazenil, a benzodiazepine antagonist, may precipitate acute withdrawal reactions, including seizures, which can be life-threatening. Patients at an increased risk of withdrawal adverse reactions after benzodiazepine discontinuation or rapid dosage reduction include those who take higher dosages (i.e., higher and/or more frequent doses) and those who have had longer durations of use (see WARNINGS, Dependence and Withdrawal Reactions).
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue estazolam or reduce the dosage (see DOSAGE and ADMINISTRATION, Discontinuation or Dosage Reduction of Estazolam and WARNINGS, Dependence and Withdrawal Reactions).
Acute Withdrawal Signs and Symptoms
Acute withdrawal signs and symptoms associated with benzodiazepines have included abnormal involuntary movements, anxiety, blurred vision, depersonalization, depression, derealization, dizziness, fatigue, gastrointestinal adverse reactions (e.g., nausea, vomiting, diarrhea, weight loss, decreased appetite), headache, hyperacusis, hypertension, irritability, insomnia, memory impairment, muscle pain and stiffness, panic attacks, photophobia, restlessness, tachycardia, and tremor. More severe acute withdrawal signs and symptoms, including life-threatening reactions, have included catatonia, convulsions, delirium tremens, depression, hallucinations, mania, psychosis, seizures, and suicidality.
Protracted Withdrawal Syndrome
Protracted withdrawal syndrome associated with benzodiazepines is characterized by anxiety, cognitive impairment, depression, insomnia, formication, motor symptoms (e.g., weakness, tremor, muscle twitches), paresthesia, and tinnitus that persists beyond 4 to 6 weeks after initial benzodiazepine withdrawal. Protracted withdrawal symptoms may last weeks to more than 12 months. As a result, there maybe difficulty in differentiating withdrawal symptoms from potential re-emergence or continuation of symptoms for which the benzodiazepine was being used.
Tolerance
Tolerance to estazolam may develop from continued therapy. Tolerance is a physiological state characterized by a reduced response to a drug after repeated administration (i.e., a higher dose of a drug is required to produce the same effect that was once obtained at a lower dose). Tolerance to the therapeutic effect of estazolam may develop estazolam; however, little tolerance develops to the amnestic reactions and other cognitive impairments caused by benzodiazepines.
-
OVERDOSAGE
Overdosage of benzodiazepines is characterized by central nervous system depression ranging from drowsiness to coma. In mild to moderate cases, symptoms can include drowsiness, confusion, dysarthria, lethargy, hypnotic state, diminished reflexes, ataxia, and hypotonia. Rarely, paradoxical or disinhibitory reactions (including agitation, irritability, impulsivity, violent behavior, confusion, restlessness, excitement, and talkativeness) may occur. In severe overdosage cases, patients may develop respiratory depression and coma. Overdosage of benzodiazepines in combination with other CNS depressants (including alcohol and opioids) may be fatal (see WARNINGS: Dependence and Withdrawal Reactions). Markedly abnormal (lowered or elevated) blood pressure, heart rate, or respiratory rate raise the concern that additional drugs and/or alcohol are involved in the overdosage.
In managing benzodiazepine overdosage, employ general supportive measures, including intravenous fluids and airway management. Flumazenil, a specific benzodiazepine receptor antagonist, is indicated for the complete or partial reversal of the sedative effects of benzodiazepines in the management of benzodiazepine overdosage, can lead to withdrawal and adverse reactions, including seizures, particularly in the context of mixed overdosage with drugs that increase seizure risk (e.g. tricyclic and tetracyclic antidepressants) and in patients with long-term benzodiazepine use and physical dependency. The risk of withdrawal seizures with flumazenil use may be increased in patients with epilepsy. Flumazenil is contraindicated in patients who have received a benzodiazepine for control of potentially life-threatening condition (e.g. status epilepticus). If the decision is made to use flumazenil, it should be used as an adjunct to, not as a substitute for, supportive management of benzodiazepine overdosage. See the flumazenil injection Prescribing Information.
Consider contacting a poison center (1-800-222-2222), poisoncontrol.org, or a medical toxicologist for additional overdosage management recommendations.
-
DOSAGE AND ADMINISTRATION
The recommended initial dose for adults is 1 mg at bedtime; however, some patients may need a 2 mg dose. In healthy elderly patients, 1 mg is also the appropriate starting dose, but increases should be initiated with particular care. In small or debilitated older patients, a starting dose of 0.5 mg, while only marginally effective in the overall elderly population, should be considered.
Discontinuation or Dosage Reduction of Estazolam
To reduce the risk of withdrawal reactions, use a gradual taper to discontinue estazolam or reduce the dosage. If a patient develops withdrawal reactions, consider pausing the taper or increasing the dosage to the previous tapered dosage level. Subsequently decrease the dosage more slowly (see WARNINGS, Dependence and Withdrawal Reactions and DRUG ABUSE AND DEPENDENCE, Dependence).
-
HOW SUPPLIED
Estazolam tablets 1 mg are available as white, oval, convex tablet, bisected and debossed "m" and "073" on one side, bisected and plain on the other side. Packaged in bottles of 100 (NDC: 75907-028-01).
Estazolam tablets 2 mg are available as pale orange, oval, convex tablet, bisected and debossed "m" and "074" on one side, plain on the other side. Packaged in bottles of 100 (NDC: 75907-029-01).
- SPL UNCLASSIFIED SECTION
-
MEDICATION GUIDE
MEDICATION GUIDE
Estazolam (es-TAZE-oh-lam) Tablets, C-IVThis Medication Guide has been approved by the U.S. Food and Drug Administration Rev. 08/23 What is the most important information I should know about estazolam? -
Estazolam is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (CNS) (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
- shallow or slowed breathing
- breathing stops (which may lead to the heart stopping)
- excessive sleepiness (sedation)
-
Risk of abuse, misuse, and addiction. There is a risk of abuse, misuse, and addiction with benzodiazepines, including estazolam, which can lead to overdose and serious side effects including coma and death.
- Serious side effects including coma and death have happened in people who have abused or misused benzodiazepines, including estazolam. These serious side effects may also include delirium, paranoia, suicidal thoughts or actions, seizures, and difficulty breathing. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these serious side effects.
- You can develop an addiction even if you take estazolam exactly as prescribed by your healthcare provider.
- Take estazolam exactly as your healthcare provider prescribed.
- Do not share your estazolam with other people.
- Keep estazolam in a safe place and away from children.
-
Physical dependence and withdrawal reactions. Estazolam can cause physical dependence and withdrawal reactions, especially if you continue to take estazolam for several days to several weeks.
- Do not suddenly stop taking estazolam. Stopping estazolam suddenly can cause serious and life-threatening side effects, including unusual movements, responses, or expressions, seizures, sudden and severe mental or nervous system changes, depression, seeing or hearing things that others do not see or hear, an extreme increase in activity or talking, losing touch with reality, and suicidal thoughts or actions. Call your healthcare provider or go to the nearest hospital emergency room right away if you get any of these symptoms.
- Some people who suddenly stop benzodiazepines have symptoms that can last for several weeks to more than 12 months, including, anxiety, trouble remembering, learning, or concentrating, depression, problems sleeping, feeling like insects are crawling under your skin, weakness, shaking, muscle twitching, burning or prickling feeling in your hands, arms, legs or feet, and ringing in your ears.
- Physical dependence is not the same as drug addiction. Your healthcare provider can tell you more about the differences between physical dependence and drug addiction.
- Do not take more estazolam than prescribed or take estazolam for longer than prescribed.
- After taking estazolam, you may get up out of bed while not being fully awake and do an activity that you do not know you are doing. The next morning, you may not remember that you did anything during the night. You have a higher chance for doing these activities if you drink alcohol or take other medicines that make you sleepy with estazolam. Reported activities include:
- driving a car ("sleep-driving")
- making and eating food
- talking on the phone
- having sex
- sleep-walking
Call your healthcare provider right away if you find out that you have done any of the above activities after taking estazolam. - Do not take estazolam unless you are able to stay in bed a full night (7 to 8 hours) before you must be active again.
What is estazolam? - Estazolam is a prescription medicine used short-term to treat certain types of insomnia including difficulty falling asleep, waking up often during the night, or waking up early in the morning.
- Estazolam is a federal controlled substance (C-IV) because it contains estazolam that can be abused or lead to dependence. Keep estazolam in a safe place to prevent misuse and abuse. Selling or giving away estazolam may harm others, and is against the law. Tell your healthcare provider if you have ever abused or been dependent on alcohol, prescription medicines or street drugs.
- It is not known if estazolam is safe and effective in children.
- It is not known if estazolam is safe and effective when used to treat insomnia for longer than 12 weeks.
Do not take estazolam if you: - are allergic to estazolam, other benzodiazepines, or any of the ingredients in estazolam. See the end of this Medication Guide for a complete list of ingredients in estazolam.
- take antifungal medicines called ketoconazole and itraconazole
Before you take estazolam, tell your healthcare provider about all of your medical conditions, including if you: - have a history of depression, mental illness or, suicidal thoughts
- have a history of drug or alcohol abuse or addiction
- have kidney or liver problems
- have lung disease or breathing problems
- are pregnant or plan to become pregnant.
- Taking estazolam late in pregnancy may cause your baby to have symptoms of sedation (breathing problems, sluggishness, low muscle tone), and/or withdrawal symptoms (jitteriness, irritability, restlessness, shaking, excessive crying, feeding problems).
- Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with estazolam.
- There is a pregnancy registry for women who take estazolam during pregnancy. The purpose of the registry is to collect information about the health of you and your baby. If you become pregnant during treatment with estazolam, talk to your healthcare provider about registering with the National Pregnancy Registry for Psychiatric Medications. You can register by calling 1-866-961-2388 or visiting https://womensmentalhealth.org/pregnancyregistry/.
- are breastfeeding, or plan to breastfeed.
- Talk to your healthcare provider about the best way to feed your baby if you take estazolam.
- Breastfeeding is not recommended during treatment with estazolam.
Tell your healthcare provider about all of the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Taking estazolam with certain other medicines can cause side effects or affect how well estazolam or the other medicines work. Do not start or stop other medicines without talking to your healthcare provider. Do not take estazolam with other medicines that can make you sleepy unless your healthcare provider tells you to. How should I take estazolam? - See "What is the most important information I should know about estazolam?"
- Take estazolam exactly as your healthcare provider tells you to take it.
- Take estazolam right before you get into bed. Or you can take estazolam after you have been in bed and have trouble falling asleep.
- Do not take estazolam with or right after a meal.
- Do not take estazolam unless you are able to get a full night's sleep before you must be active again.
- If you take too much estazolam, get emergency treatment right away.
What are the possible side effects of estazolam? Estazolam may cause serious side effects, including: - See "What is the most important information I should know about estazolam?
- Other conditions. Call your healthcare provider if your insomnia worsens or is not better within 7 to 10 days. This may mean that there is another condition causing your sleep problem.
- Severe allergic reactions. Symptoms include swelling of the tongue or throat, and trouble breathing. Get emergency medical help right away if you have these symptoms after taking estazolam.
- Abnormal thoughts and behavior. Symptoms include more outgoing or aggressive behavior than normal, confusion, agitation, hallucinations, worsening of depression, and suicidal thoughts.
-
Estazolam can make you sleepy or dizzy and can slow your thinking and motor skills.
- Do not drive, operate heavy machinery, or do other dangerous activities until you know how estazolam affects you.
- Do not drink alcohol or take other medicines that may make you sleepy or dizzy while taking estazolam without first talking to your healthcare provider. When taken with alcohol or medicines that cause sleepiness or dizziness, estazolam may make your sleepiness or dizziness much worse.
The most common side effects of estazolam include: - drowsiness
- dizziness
- headache
- dry mouth
- fatigue
- upset stomach
You may still feel drowsy the next day after taking ESTAZOLAM. Do not drive or do other dangerous activities after taking ESTAZOLAM until you feel fully awake. These are not all the possible side effects of estazolam. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. How should I store estazolam? - Store estazolam tablets in a tightly closed child resistant container at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep estazolam and all medicines out of the reach of children.
General information about the safe and effective use of estazolam. Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use estazolam for a condition for which it was not prescribed. Do not give estazolam to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about estazolam that is written for healthcare professionals. What are the ingredients in estazolam? Active Ingredient: estazolam Inactive Ingredients: 1 mg tablets - corn starch, lactose monohydrate, pregelatinized starch, and stearic acid; 2 mg tablets - corn starch, FD&C Red #40 aluminum lake, FD&C Yellow #6 aluminum lake, lactose monohydrate, pregelatinized starch, and stearic acid.
Distributed by:
Dr. Reddy’s Laboratories Inc.
Princeton, NJ 08540 USAIf you would like more information, call Dr. Reddy’s Laboratories at 1-888-375-3784. -
Estazolam is a benzodiazepine medicine. Taking benzodiazepines with opioid medicines, alcohol, or other central nervous system depressants (CNS) (including street drugs) can cause severe drowsiness, breathing problems (respiratory depression), coma and death. Get emergency help right away if any of the following happens:
-
PRINCIPAL DISPLAY PANEL - 1 mg Tablet Bottle Label
NDC: 75907-028-01
Estazolam
Tablets
CIV1 mg
PHARMACIST: Dispense the
accompanying Medication
Guide to each patient.Rx Only
100 Tablets
Dr. Reddy's Laboratories, Inc

-
PRINCIPAL DISPLAY PANEL - 2 mg Tablet Bottle Label
NDC: 75907-029-01
Estazolam
Tablets
CIV2 mg
PHARMACIST: Dispense the
accompanying Medication
Guide to each patient.Rx Only
100 Tablets
Dr. Reddy's Laboratories, Inc

-
INGREDIENTS AND APPEARANCE
ESTAZOLAM
estazolam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75907-028 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Estazolam (UNII: 36S3EQV54C) (Estazolam - UNII:36S3EQV54C) Estazolam 1 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STEARIC ACID (UNII: 4ELV7Z65AP) Product Characteristics Color WHITE Score 2 pieces Shape OVAL Size 7mm Flavor Imprint Code m;073 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75907-028-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074921 02/15/2024 ESTAZOLAM
estazolam tabletProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 75907-029 Route of Administration ORAL DEA Schedule CIV Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Estazolam (UNII: 36S3EQV54C) (Estazolam - UNII:36S3EQV54C) Estazolam 2 mg Inactive Ingredients Ingredient Name Strength STARCH, CORN (UNII: O8232NY3SJ) LACTOSE MONOHYDRATE (UNII: EWQ57Q8I5X) STEARIC ACID (UNII: 4ELV7Z65AP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color ORANGE (pale orange) Score 2 pieces Shape OVAL Size 9mm Flavor Imprint Code m;074 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 75907-029-01 100 in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 02/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA074921 02/15/2024 Labeler - Dr. Reddy's Labratories Inc. (802315887)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.