Guaifenesin by American Health Packaging GUAIFENESIN solution
Guaifenesin by
Drug Labeling and Warnings
Guaifenesin by is a Otc medication manufactured, distributed, or labeled by American Health Packaging. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- cough that occurs with too much phlegm (mucus)
- cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis, or emphysema
-
Directions
Follow dosage below or use as directed by a physician.
- do not take more than 6 doses in any 24-hour period.
Age (yr)
Dose (ml)
adults and children 12 years and over
10 to 20 ml (2 to 4 teaspoonfuls) every 4 hours
children 6 years to under 12 years
5 to 10 ml (1 to 2 teaspoonfuls) every 4 hours
children 2 years to under 6 years
2.5 to 5 ml (1 /2 to 1 teaspoonful) every 4 hours
children under 2 years
consult a physician
Other Information
- Each 5 ml contains: sodium 4 mg.
- Each 10 ml contains: sodium 8 mg.
- Each 15 ml contains: sodium 12 mg.
- Store at controlled room temperature between 20° to 25°C (68° to 77°F) [see USP]. Protect from light.
- DO NOT USE IF SEAL IS BROKEN.
- Guaifenesin Oral Solution is a red, raspberry flavored solution and is available in the following dosage forms:
5 ml unit-dose cups: 100 cups (10 x 10) NDC: 60687-852-17
10 ml unit-dose cups: 100 cups (10 x 10) NDC: 60687-863-56
15 ml unit-dose cups: 100 cups (10 x 10) NDC: 60687-874-16
- Inactive Ingredients
- Questions or comments?
- Generic Section
- Package/Label Principal Display Panel – 100 mg per 5 mL – Label
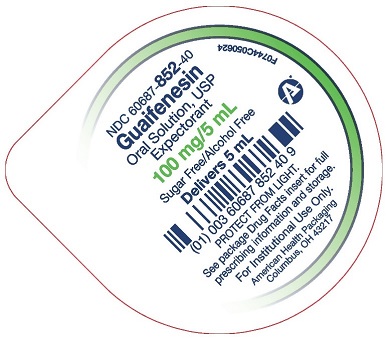
- Package/Label Principal Display Panel – 100 mg per 5 mL – Cup Lid
-
Package/Label Principal Display Panel – 200 mg per 10 mL – Label

Case NDC 60687- 863-56/Cup NDC 60687- 863-42
Guaifenesin
Oral Solution USP
Expectorant200 mg per 10 ml
Sodium Content: 8 mg/10 ml
SUGAR FREE / ALCOHOL FREE
Raspberry FlavorUsual Dosage: See attached Drug Facts.
Store at 20° to 25°C (68° to 77°F) [See USP]
Protect from light.FOR INSTITUTIONAL USE ONLY
T0744C100624 R0624
-
Package/Label Principal Display Panel – 200 mg per 10 mL – Cup Lid

NDC 60687- 863-42
Guaifenesin
Oral Solution, USP
Expectorant200 mg per 10 ml
Sodium Content: 8 mg/10 ml
Sugar Free/Alcohol Free
Delivers 10 mLPROTECT FROM LIGHT.
See package Drug Facts insert for full
prescribing information and storage.For Institutional Use Only.
American Health Packaging
Columbus, OH 43217F0744C100624
-
Package/Label Principal Display Panel – 300 mg per 15 mL – Label

Case NDC 60687- 874-16/Cup NDC 60687- 874-44
Guaifenesin
Oral Solution USP
Expectorant300 mg per 15 ml
Sodium Content: 12 mg/15 ml
SUGAR FREE / ALCOHOL FREE
Raspberry FlavorUsual Dosage: See attached Drug Facts.
Store at 20° to 25°C (68° to 77°F) [See USP]
Protect from light.FOR INSTITUTIONAL USE ONLY
T0744C150624 R0624
-
Package/Label Principal Display Panel – 300 mg per 15 mL – Cup Lid
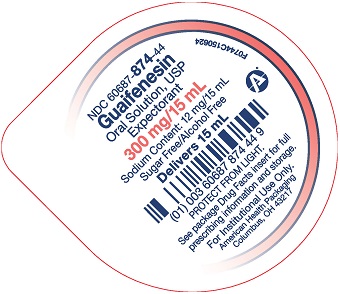
NDC 60687- 874-44
Guaifenesin
Oral Solution, USP
Expectorant300 mg per 15 ml
Sodium Content: 12 mg/15 ml
Sugar Free/Alcohol Free
Delivers 15 mLPROTECT FROM LIGHT.
See package Drug Facts insert for full
prescribing information and storage.For Institutional Use Only.
American Health Packaging
Columbus, OH 43217F0744C150624
-
INGREDIENTS AND APPEARANCE
GUAIFENESIN
guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60687-852 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) Product Characteristics Color red Score Shape Size Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60687-852-17 10 in 1 CASE 10/20/2024 1 NDC: 60687-852-46 10 in 1 TRAY 1 NDC: 60687-852-40 5 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/20/2024 GUAIFENESIN
guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60687-863 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 200 mg in 10 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Product Characteristics Color red Score Shape Size Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60687-863-56 10 in 1 CASE 10/20/2024 1 NDC: 60687-863-48 10 in 1 TRAY 1 NDC: 60687-863-42 10 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/20/2024 GUAIFENESIN
guaifenesin solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 60687-874 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 300 mg in 15 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) HYDROXYETHYL CELLULOSE, UNSPECIFIED (UNII: T4V6TWG28D) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CITRATE, UNSPECIFIED FORM (UNII: 1Q73Q2JULR) Product Characteristics Color red Score Shape Size Flavor RASPBERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 60687-874-16 10 in 1 CASE 10/20/2024 1 NDC: 60687-874-50 10 in 1 TRAY 1 NDC: 60687-874-44 15 mL in 1 CUP, UNIT-DOSE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 10/20/2024 Labeler - American Health Packaging (929561009)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.