HEMOCYTE F- folic acid and iron tablet
Hemocyte F by
Drug Labeling and Warnings
Hemocyte F by is a Other medication manufactured, distributed, or labeled by US Pharmaceutical Corporation, PD SUB, LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- STATEMENT OF IDENTITY
- STATEMENT OF IDENTITY
- STATEMENT OF IDENTITY
-
STATEMENT OF IDENTITY
CONTRAINDICATIONS: Hemocyte-F is contraindicated in patients with a known hypersensitivity to any of its ingredients; also, all iron compounds are contraindicated in patients with hemosiderosis, hemochromatosis, or hemolytic anemias. Pernicious anemia is a contraindication, as folic acid may obscure its signs and symptoms.
-
WARNINGS
WARNING: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of reach of children. In case of accidental overdose, call a doctor or poison control center immediately. WARNING:Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where Vitamin B12 is deficient.
You should contact your healthcare provider for medical advice about adverse events. To report a serious adverse event, contact US Pharmaceutical Corporation, P.O. Box 360465,Decatur,GA 30036
Ferrous Fumarate : 2-Butenediac acid (E)-, iron (2+) salt is a hematinic which occurs as a reddish orange to red brown, odourless powder. it may contain soft lumps that produce a yellow streak when crushed. It is slightly soluble in water and very slightly soluble in alcohol. Its solubility in dilute hydrochloric acid is limited by the separation of fumaric acid .Its molecular formula and Chemical structure follows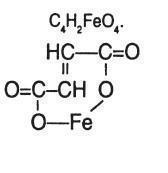
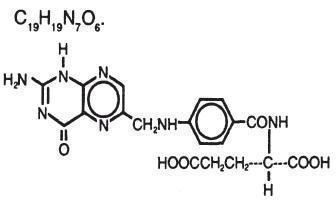
Folic Acid :N-(4-{{(2-amino-1,4-dihydro-4-oxo-6-petridinyl) methyl) amino benzoyl}-1 glutamic acid , is a vitamin (hematopoietic) which occurs as a yellow , yellowish brown ,or yellowish orange ,odorless crystalline powder which is very slightly soluble in water ,insoluble in alcohol, acetone, chloroform, or ether. It dissolves readily in dilute solutions of alkali hydroxides and carbonates and is soluble in hot 3 N hydrochloric acid and in hot , 2N sulfuric acid , yielding very pale yellow solutions. Its molecular formula and chemical structure follow.
CLINICAL PHARMACLOGY: This product is formulated to meet the needs of patient requiring both iron and folic acid. Deficiencies of these ingredients are common during pregnancy. Ferrous fumarate contains approximately 33 percent elemental Iron. The use of well tolerated ferrous fumarate provides a high level of elemental iron with a low incidence of gastric distress.
Ferrous Fumarate: A very common anemia is that due to iron deficiency. in most cases the response to iron salts is prompt, safe and predictable. Within limits the response is quicker and more certain to large doses of iron than to small doses. Iron is absorbed from the small intestine ;however, the exact mechanism regulating the amount absorbed is still a matter of controversy. The proportion of dietary iron absorbed is greater in iron deficient anemic individuals. Iron is transported via the blood in which it is bound to transferrin, a beta -1-globulin.The iron from deteriorated red blood cells is reutilized. Under normal circumstances the loss of iron from the body is very small, about 1 mg per day for men and an additional average daily loss of 0.5 mg/day by menstruating women. Iron is stored in the bone marrow, intestinal wall, liver and spleen with the latter organs containing the largest amounts.
Folic Acid: Folic Acid is one of the important hematopoetic agents necessary for proper regeneration of the blood-forming elements and their function. Folic acid is a precursor of a large family of compounds which serve as coenzymes in carbon transfer reactions. These reactions are required for the synthesis of purine and pyrimidine bases, inter-conversion of glycine and serine, biosynthesis of methionine methyl groups and degradation of histidine. Additionally, folic acid increases jejunal glycolytic enzymes and is involved in the desaturation and hydroxylation of long-chain fatty acids in the brain. A deficiency in folic acid results in megaloblastic anemia.
-
PRECAUTIONS
PRECAUTIONS: General: Anemia is a manifestation that requires appropriate investigation to determine its cause or causes. No single regimen fits all cases and the status of the patient observed in follow-up is the final criterion for adequacy of therapy. Periodic clinical and laboratory studies are considered essential. Blood examinations including hemoglobin and hematocrit should be done at the usual intervals to make certain that therapy is adequate. Use with care in the presence of peptic ulcer, regional enteritis, and ulcerative colitis. Folic acid, especially in doses above 0.1 mg -0.4 mg daily may obscure pernicious anemia, in that hematologic remission can occur while neurological manifestations remain progressive.
Usage in Pregnancy: Before Integra FTM is prescribed for megaloblastic anemia in pregnancy, appropriate diagnostic exclusion of Addisonian pernicious anemia, (due to faulty or blocked absorption of vitamin B12, or extrinsic factor or either a genetic, immunological or surgical basis) should be carried out.
Pediatric Use: Safety and effectiveness of this product have not been established in pediatric patients.Geriatric Use: No clinical studies have been performed in patients 65 and over to determine whether older persons respond differently from younger persons. Dosage should always begin at the low end of the dosage scale and should consider that elderly persons may have decreased hepatic, renal, or cardiac function and or concomitant diseases.
ADVERSE REACTIONS: Ferrous Fumarate has been implicated in some instances of gastrointestinal disturbances including abdominal cramps, diarrhea, constipation, anorexia, heartburn, nausea, and vomiting. Reducing the dose and administering it with meals will minimize these effects in the sensitive patient. Increasing fiber in the diet can relieve constipation. Iron may turn stools black. This is a harmless effect that is a result of unabsorbed iron. Allergic sensitisation has been reported following oral administration of folic acid.
OVERDOSAGE: Iron: Signs and Symptoms: Iron is toxic. Acute overdosage of iron may cause nausea and vomiting and, in severe cases, cardiovascular collapse and death. Other symptoms include pallor and cyanosis, melena, shock, drowsiness and coma. The estimated lethal dose of orally ingested elemental iron is 300 mg per kg body weight. Hemocyte -F should be stored beyond the reach of children to protect against accidental iron poisoning. Keep this product and all other drugs out of the reach of children.
Treatment: For specific therapy, exchange transfusion and chelating agents should be used. For general management, perform gastric lavage with sodium bicarbonate solution or milk, administer intravenous fluids and electrolytes and use oxygen.
- SAFE HANDLING WARNING
-
DOSAGE & ADMINISTRATION
Usual adult dose is one tablet taken orally daily or as prescribed by a physician.
HOW SUPPLIED: Hemocyte-F are maroon film coated round tablets debossed with "US" logo on one side and "F" on the other side. Hemocyte-F are supplied in child resistant unit dose packs of 100 tablets and 30 tablets .Boxes of 100s: NDC: 52747-306-70, boxes of 30s: NDC: 52747-306-30. Dispense in a tight, light-resistant container as defined in the USP/NF with a child resistant closure.
- HEALTH CLAIM
- Packaging
-
INGREDIENTS AND APPEARANCE
HEMOCYTE F
folic acid and iron tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:52747-306 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1 mg FERROUS FUMARATE (UNII: R5L488RY0Q) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 106 mg Inactive Ingredients Ingredient Name Strength CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) SODIUM STARCH GLYCOLATE TYPE A POTATO (UNII: 5856J3G2A2) POLYVINYL ALCOHOL (UNII: 532B59J990) MAGNESIUM STEARATE (UNII: 70097M6I30) POLYETHYLENE GLYCOLS (UNII: 3WJQ0SDW1A) FD&C RED NO. 40 (UNII: WZB9127XOA) TALC (UNII: 7SEV7J4R1U) HYPROMELLOSES (UNII: 3NXW29V3WO) MALTODEXTRIN (UNII: 7CVR7L4A2D) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:52747-306-70 10 in 1 BOX 1 10 in 1 BLISTER PACK Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 10/01/1984 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 10 mm scoring 1 imprint Labeler - US Pharmaceutical Corporation (048318224) Establishment Name Address ID/FEI Business Operations PD SUB, LLC. 063753431 manufacture(52747-306)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
