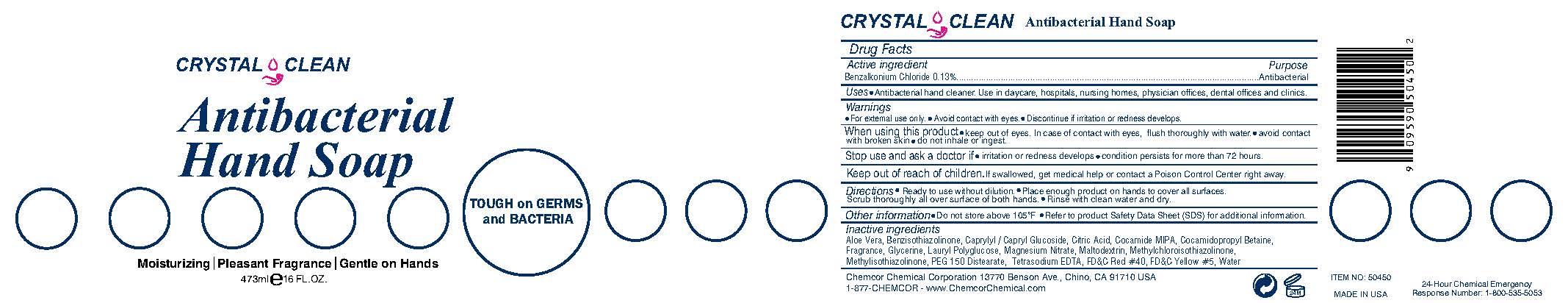
CRYSTAL CLEAN ANTIBACTERIAL HANDSOAP- benzalkonium chloride liquid
Crystal Clean Antibacterial HandSoap by
Drug Labeling and Warnings
Crystal Clean Antibacterial HandSoap by is a Otc medication manufactured, distributed, or labeled by Chemcor Chemical Corporation, Morgan Gallacher Inc. DBA Custom Chemical Formulators Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient
- Purpose
- INDICATIONS & USAGE
-
Warnings
For external use only. Avoid contact with eyes. Discontinue if irritation or redness develops.
When using this product keep out of eyes. In case of contact with eyes, flush thoroughly with water. avoid contact with broken skin do not inhale or ingest.
Stop use and ask a doctor if irritation or redness develops condition persists for more than 72 hours.
- DOSAGE & ADMINISTRATION
-
Inactive ingredients
Aloe Barbadensis Leaf Juice, Caprylyl/Capryl Glucoside, Citric Acid, Cocamide MIPA, Cocamidopropyl Betaine, Fragrance, Glycerin, Lauryl Polyglucose, Magnesium Nitrate, Maltodextrin, Methylchloroisothiazolinone, Methylisothiazolinone, PEG 150 Distearate, FD&C Red #40, Tetrasodium EDTA, Water, FD&C Yellow #5
-
SPL UNCLASSIFIED SECTION
TOUGH on GERMS and BACTERIA
Moisturizing | Pleasant Fragrance | Gentle on Hands
May be harmful if swallowed. May cause irritation to eyes.
Chemcor Chemical Corporation 13770 Benson Ave., Chino, CA 91710 USA
1-877-CHEMCOR - www.ChemcorChemical.com
MADE IN USA
24-Hour Chemical Emergency
Response Number: 1-800-535-5053
- Crystal-Clean Antibacterial Hand Soap
-
INGREDIENTS AND APPEARANCE
CRYSTAL CLEAN ANTIBACTERIAL HANDSOAP
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 68041-740 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE .13 g in 1 mL Inactive Ingredients Ingredient Name Strength ALOE VERA LEAF (UNII: ZY81Z83H0X) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) COCO MONOISOPROPANOLAMIDE (UNII: 21X4Y0VTB1) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) GLYCERIN (UNII: PDC6A3C0OX) LAURYL GLUCOSIDE (UNII: 76LN7P7UCU) MAGNESIUM NITRATE (UNII: 77CBG3UN78) MALTODEXTRIN (UNII: 7CVR7L4A2D) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) PEG-150 DISTEARATE (UNII: 6F36Q0I0AC) FD&C RED NO. 40 (UNII: WZB9127XOA) EDETATE SODIUM (UNII: MP1J8420LU) WATER (UNII: 059QF0KO0R) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 68041-740-50 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 02/02/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M 02/02/2023 Labeler - Chemcor Chemical Corporation (018129978) Establishment Name Address ID/FEI Business Operations Morgan Gallacher Inc. DBA Custom Chemical Formulators Inc. 028311595 manufacture(68041-740) , api manufacture(68041-740) , pack(68041-740)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.