CALCEMIN ADVANCE- calcium citrate tablet, film coated
Calcemin Advance by
Drug Labeling and Warnings
Calcemin Advance by is a Otc medication manufactured, distributed, or labeled by Bayer HealthCare LLC.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
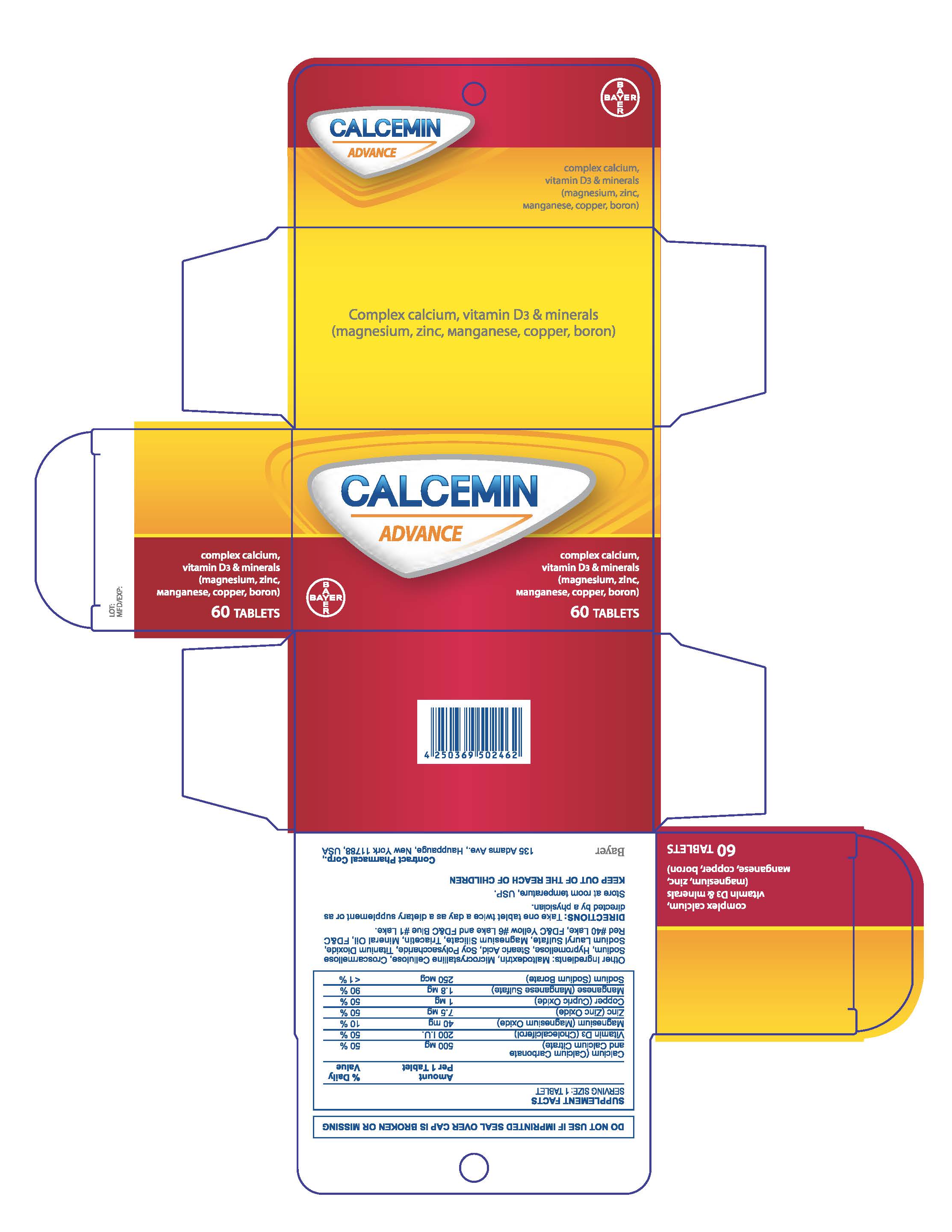
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CALCEMIN ADVANCE
calcium citrate tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 0280-0272 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM CARBONATE (UNII: H0G9379FGK) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM CARBONATE 500 mg PREVITAMIN D3 (UNII: HDA46400N5) (PREVITAMIN D3 - UNII:HDA46400N5) PREVITAMIN D3 3 mg MAGNESIUM OXIDE (UNII: 3A3U0GI71G) (MAGNESIUM CATION - UNII:T6V3LHY838) MAGNESIUM OXIDE 40 mg ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 7.5 mg SODIUM BORATE (UNII: 91MBZ8H3QO) (BORATE ION - UNII:44OAE30D22) SODIUM BORATE 2.45 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) STEARIC ACID (UNII: 4ELV7Z65AP) SODIUM LAURYL SULFATE (UNII: 368GB5141J) HYPROMELLOSES (UNII: 3NXW29V3WO) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) MAGNESIUM SILICATE (UNII: 9B9691B2N9) MINERAL OIL (UNII: T5L8T28FGP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) WATER (UNII: 059QF0KO0R) MALTODEXTRIN (UNII: 7CVR7L4A2D) Product Characteristics Color white Score no score Shape OVAL Size 15mm Flavor Imprint Code None Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0280-0272-30 1 in 1 CARTON 04/01/2016 1 30 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC: 0280-0272-60 1 in 1 CARTON 04/01/2016 2 60 in 1 BOTTLE; Type 0: Not a Combination Product 3 NDC: 0280-0272-12 1 in 1 CARTON 04/01/2016 3 120 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 04/01/2016 Labeler - Bayer HealthCare LLC. (112117283)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.