SLEEP STARTER TOPICAL PATCH,Sleep Patch
SLEEP STARTER TOPICAL Patch, Sleep Patch by
Drug Labeling and Warnings
SLEEP STARTER TOPICAL Patch, Sleep Patch by is a Otc medication manufactured, distributed, or labeled by ZheJiang Longmed Medical Technology Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
SLEEP STARTER TOPICAL PATCH, SLEEP PATCH- melatonin, valeriana officinalis patch
ZheJiang Longmed Medical Technology Co., Ltd.
----------
SLEEP STARTER TOPICAL PATCH,Sleep Patch
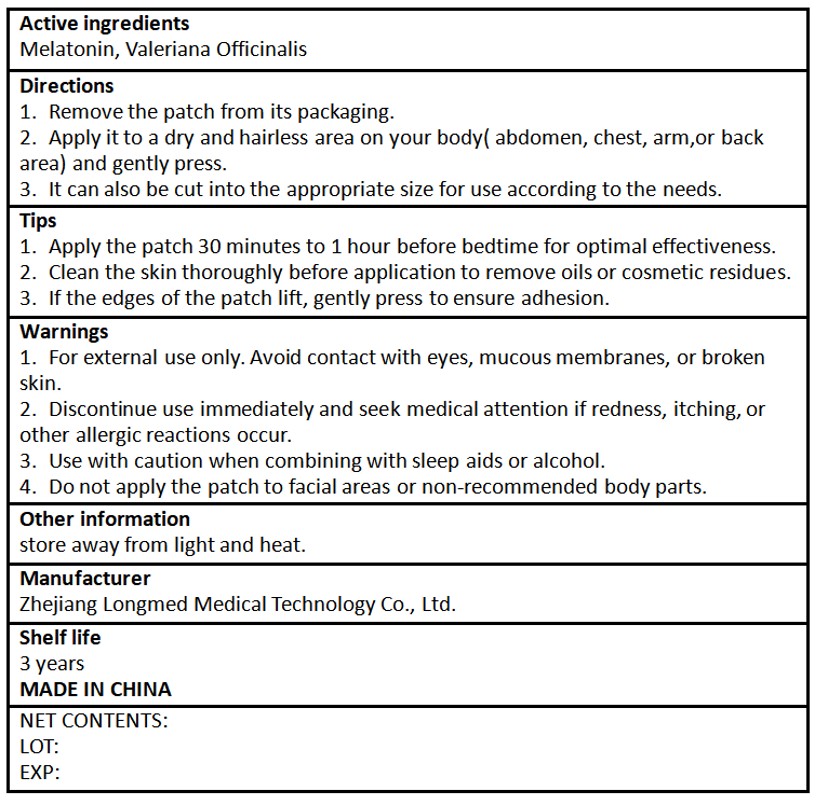
1. For external use only. Avoid contact with eyes, mucous membranes, or brokenskin.
2. Discontinue use immediately and seek medical attention if redness, itching, orother allergic reactions occur.
3. Use with caution when combining with sleep aids or alcohol.
4. Do not apply the patch to facial areas or non-recommended body parts.
1. Remove the patch from its packaging.
2. Apply it to a dry and hairless area on your body( abdomen, chest, arm,or backarea) and gently press.
3. it can also be cut into the appropriate size for use according to the needs.
| SLEEP STARTER TOPICAL PATCH, SLEEP PATCH
melatonin, valeriana officinalis patch |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - ZheJiang Longmed Medical Technology Co., Ltd. (554468373) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| ZheJiang Longmed Medical Technology Co., Ltd. | 554468373 | manufacture(84534-005) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.