DIPHENHYDRAMINE HYDROCHLORIDE capsule
Diphenhydramine Hydrochloride by
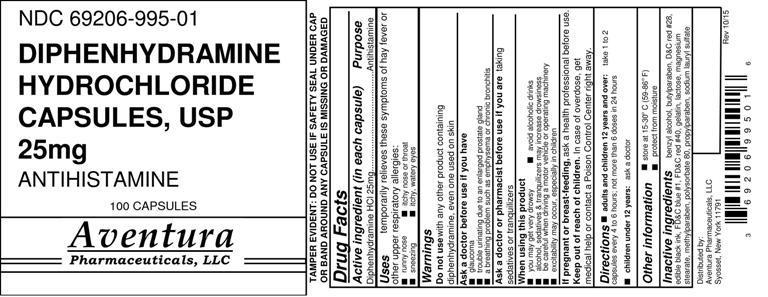
Drug Labeling and Warnings
Diphenhydramine Hydrochloride by is a Otc medication manufactured, distributed, or labeled by Aventura Pharmaceuticals, LLC, Denton Pharma, Inc. DBA Northwind Pharmaceuticals. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Drug Facts
- Active Ingredient
- Purpose
- Uses
-
WARNINGS
Do not use with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- glaucoma
- trouble urinating due to an enlarged prostate gland
- a breathing problem such as emphysema or chronic bronchitis
Ask a doctor or pharmacist before use if you are taking sedatives or tranquilizers
When using this product
- you may get very drowsy
- avoid alcoholic drinks
- alcohol, sedatives & tranquilizers may increase drowsiness
- be careful when driving a motor vehicle or operating machinery
- excitability may occur, especially in children
If pregnant or breast-feeding, ask a health professional before use.
- Keep out of reach of children.
- Directions
- Other Information
- Inactive Ingredients
- Questions or Comments
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HYDROCHLORIDE
diphenhydramine hydrochloride capsuleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 69206-995(NDC: 0603-3339) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 25 mg Inactive Ingredients Ingredient Name Strength BENZYL ALCOHOL (UNII: LKG8494WBH) BUTYLPARABEN (UNII: 3QPI1U3FV8) D&C RED NO. 28 (UNII: 767IP0Y5NH) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN (UNII: 2G86QN327L) LACTOSE (UNII: J2B2A4N98G) MAGNESIUM STEARATE (UNII: 70097M6I30) METHYLPARABEN (UNII: A2I8C7HI9T) POLYSORBATE 80 (UNII: 6OZP39ZG8H) PROPYLPARABEN (UNII: Z8IX2SC1OH) SODIUM LAURYL SULFATE (UNII: 368GB5141J) Product Characteristics Color PINK Score no score Shape CAPSULE Size 14mm Flavor Imprint Code AP;020 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 69206-995-01 100 in 1 BOTTLE; Type 0: Not a Combination Product 10/14/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part341 10/14/2015 Labeler - Aventura Pharmaceuticals, LLC (079493910) Registrant - Aventura Pharmaceuticals, LLC (079493910) Establishment Name Address ID/FEI Business Operations Denton Pharma, Inc. DBA Northwind Pharmaceuticals 080355546 repack(69206-995)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.