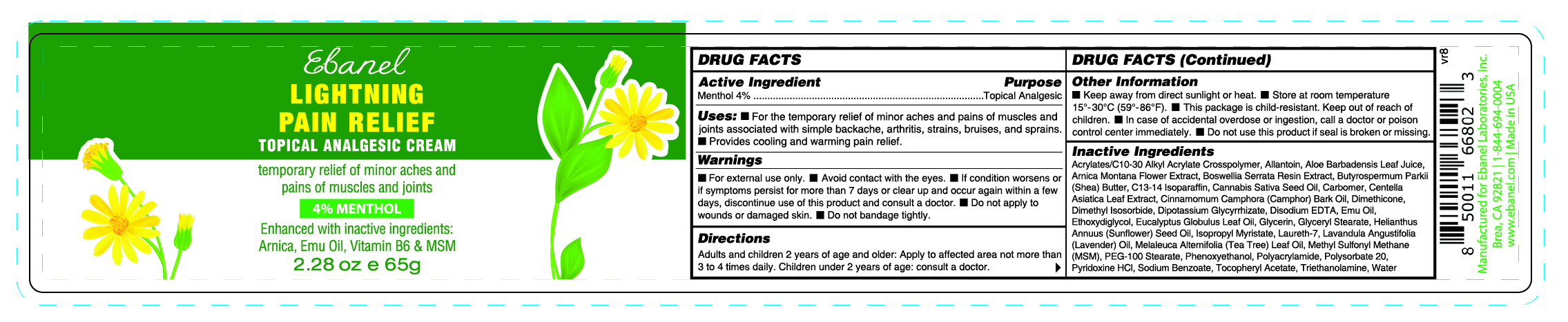
LIGHTNING PAIN RELIEF- menthol cream
Lightning Pain Relief by
Drug Labeling and Warnings
Lightning Pain Relief by is a Otc medication manufactured, distributed, or labeled by Ebanel Laboratories, Inc, Clinical Resolution Laboratory, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- PURPOSE
- DOSAGE & ADMINISTRATION
-
WARNINGS
Warnings
■ For external use only.
■ Avoid contact with the eyes.
■ If condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days, discontinue use of this product and consult a doctor.
■ Do not apply to wounds or damaged skin.
■ Do not bandage tightly.
- INDICATIONS & USAGE
-
KEEP OUT OF REACH OF CHILDREN
Other Information
■ Keep away from direct sunlight or heat.
■ Store at room temperature 15°-30°C (59°-86°F).
■ This package is child-resistant. Keep out of reach of children.
■ In case of accidental overdose or ingestion, call a doctor or poison control center immediately.
■ Do not use this product if seal is broken or missing.
-
INACTIVE INGREDIENT
Inactive Ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Allantoin, Aloe Barbadensis Leaf Juice, Arnica Montana Flower Extract, Boswellia Serrata Resin Extract, Butyrospermum Parkii (Shea) Butter, C13-14 Isoparaffin, Cannabis Sativa Seed Oil, Carbomer, Centella Asiatica Leaf Extract, Cinnamomum Camphora (Camphor) Bark Oil, Dimethicone, Dimethyl Isosorbide, Dipotassium Glycyrrhizate, Disodium EDTA, Emu Oil, Ethoxydiglycol, Eucalyptus Globulus Leaf Oil, Glycerin, Glyceryl Stearate, Helianthus Annuus (Sunflower) Seed Oil, Isopropyl Myristate, Laureth-7, Lavandula Angustifolia (Lavender) Oil, Melaleuca Alternifolia (Tea Tree) Leaf Oil, Methyl Sulfonyl Methane (MSM), PEG-100 Stearate, Phenoxyethanol, Polyacrylamide, Polysorbate 20, Pyridoxine HCl, Sodium Benzoate, Tocopheryl Acetate, Triethanolamine, Water
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
LIGHTNING PAIN RELIEF
menthol creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 72654-013 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL (UNII: L7T10EIP3A) (MENTHOL - UNII:L7T10EIP3A) MENTHOL 40 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER (UNII: 0A5MM307FC) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL (UNII: VIF565UC2G) DIMETHYL SULFONE (UNII: 9H4PO4Z4FT) PYRIDOXINE HCL (UNII: 68Y4CF58BV) ALLANTOIN (UNII: 344S277G0Z) ALOE BARBADENSIS LEAF JUICE (UNII: RUE8E6T4NB) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) BUTYROSPERMUM PARKII (SHEA) BUTTER (UNII: K49155WL9Y) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) CINNAMOMUM CAMPHORA (CAMPHOR) BARK OIL (UNII: 75IZZ8Y727) DIMETHYL ISOSORBIDE (UNII: SA6A6V432S) ETHOXYDIGLYCOL (UNII: A1A1I8X02B) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL STEARATE (UNII: 230OU9XXE4) ISOPROPYL MYRISTATE (UNII: 0RE8K4LNJS) .ALPHA.-TOCOPHEROL ACETATE, D- (UNII: A7E6112E4N) TRIETHANOLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) BOSWELLIA SERRATA RESIN OIL (UNII: 5T1XCE6K8K) CENTELLA ASIATICA LEAF (UNII: 6810070TYD) DIPOTASSIUM GLYCYRRHIZATE (UNII: CA2Y0FE3FX) EMU OIL (UNII: 344821WD61) HELIANTHUS ANNUUS (SUNFLOWER) SEED OIL (UNII: 3W1JG795YI) LAURETH-7 (UNII: Z95S6G8201) LAVANDULA ANGUSTIFOLIA (LAVENDER) OIL (UNII: ZBP1YXW0H8) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYSORBATE 20 (UNII: 7T1F30V5YH) SODIUM BENZOATE (UNII: OJ245FE5EU) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) DIMETHICONE (UNII: 92RU3N3Y1O) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) EUCALYPTUS GLOBULUS LEAF OIL (UNII: 2R04ONI662) PEG-100 STEARATE (UNII: YD01N1999R) POLYACRYLAMIDE (10000 MW) (UNII: E2KR9C9V2I) Product Characteristics Color white (Yellowish Offwhite) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 72654-013-00 65 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/06/2025 2 NDC: 72654-013-01 101 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 06/06/2025 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 06/06/2025 Labeler - Ebanel Laboratories, Inc (079352161) Establishment Name Address ID/FEI Business Operations Clinical Resolution Laboratory, Inc. 825047942 manufacture(72654-013)
Trademark Results [Lightning Pain Relief]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 LIGHTNING PAIN RELIEF 88839403 not registered Live/Pending |
Lee, Justin 2020-03-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.