AMRIX- cyclobenzaprine hydrochloride capsule, extended release
AMRIX by
Drug Labeling and Warnings
AMRIX by is a Prescription medication manufactured, distributed, or labeled by Cephalon, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use AMRIX safely and effectively. See full prescribing information for AMRIX.
AMRIX® (cyclobenzaprine hydrochloride extended-release capsules), for oral use
Initial U.S. Approval: 1977INDICATIONS AND USAGE
AMRIX is a muscle relaxant indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. (1)
Limitations of Use:
DOSAGE AND ADMINISTRATION
- Recommended adult dose for most patients is 15 mg taken once daily. Some patients may require 30 mg taken once daily (2)
- Recommended to take doses at approximately same time each day (2)
- Instruct patients to swallow AMRIX capsules intact or to sprinkle capsule contents on a tablespoon of applesauce and swallow immediately without chewing (2)
- Use for periods longer than 2 or 3 weeks is not recommended (2)
DOSAGE FORMS AND STRENGTHS
- Extended-release capsules: 15 and 30 mg (3)
CONTRAINDICATIONS
- Hypersensitivity to any component of this product (4)
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation (4)
- During acute recovery phase of myocardial infarction, and in patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure (4)
- Hyperthyroidism (4)
WARNINGS AND PRECAUTIONS
- Serotonin syndrome has been reported with cyclobenzaprine when used in combination with other serotonergic drugs (5.1)
- Cyclobenzaprine is structurally related to tricyclic antidepressants which have been reported to produce adverse cardiovascular effects or CNS depressant effects (5.2)
- Use in the elderly is not recommended (5.3)
- Use in patients with hepatic impairment is not recommended (5.4)
- Use with caution in patients with a history of urinary retention, angle-closure glaucoma, increased intraocular pressure and in patients taking anticholinergic medications (5.5)
ADVERSE REACTIONS
Most common adverse reactions (incidence ≥3% in any treatment group and greater than placebo): dry mouth, dizziness, fatigue, constipation, nausea, dyspepsia, and somnolence (6)
To report SUSPECTED ADVERSE REACTIONS, contact Teva Pharmaceuticals at 1-888-483-8279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
DRUG INTERACTIONS
- MAO Inhibitors: Life-threatening interactions may occur (4, 7)
- Serotonergic Drugs: Serotonin syndrome has been reported (5.1, 7)
- CNS Depressants: Effects of alcohol, barbiturates, and other CNS depressants may be enhanced (5.2, 7)
- Tramadol: Seizure risk may be enhanced (7)
- Guanethidine: Antihypertensive effect may be blocked (7)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2019
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Serotonin Syndrome
5.2 Tricyclic Antidepressant-like Effects
5.3 Use in the Elderly
5.4 Use in Patients with Hepatic Impairment
5.5 Atropine-like Action
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Hepatic Impairment
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
10 OVERDOSAGE
10.1 Manifestations
10.2 Management
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
13.2 Animal Toxicology and/or Pharmacology
14 CLINICAL STUDIES
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
16.2 Storage and Handling
17 PATIENT COUNSELING INFORMATION
- * Sections or subsections omitted from the full prescribing information are not listed.
-
1 INDICATIONS AND USAGE
AMRIX® (cyclobenzaprine hydrochloride extended-release capsules) is indicated as an adjunct to rest and physical therapy for relief of muscle spasm associated with acute, painful musculoskeletal conditions. Improvement is manifested by relief of muscle spasm and its associated signs and symptoms, namely, pain, tenderness, and limitation of motion.
Limitations of Use:
- AMRIX should be used only for short periods (up to two or three weeks) because adequate evidence of effectiveness for more prolonged use is not available and because muscle spasm associated with acute, painful musculoskeletal conditions is generally of short duration and specific therapy for longer periods is seldom warranted.
- AMRIX has not been found effective in the treatment of spasticity associated with cerebral or spinal cord disease or in children with cerebral palsy.
-
2 DOSAGE AND ADMINISTRATION
The recommended adult dose for most patients is one (1) AMRIX 15 mg capsule taken once daily. Some patients may require up to 30 mg/day, given as one (1) AMRIX 30 mg capsule taken once daily or as two (2) AMRIX 15 mg capsules taken once daily.
- It is recommended that doses be taken at approximately the same time each day.
- Use of AMRIX for periods longer than two or three weeks is not recommended [see Indications and Usage (1)].
Instruct patients to swallow AMRIX capsules intact. Alternatively, the contents of the AMRIX capsule may be sprinkled over applesauce and then swallowed. This method is appropriate only for patients able to reliably swallow the applesauce without chewing. Other foods have not been tested and should not be substituted for applesauce. Instruct the patient to:
- Sprinkle the contents of the capsule onto a tablespoon of applesauce and consume immediately without chewing.
- Rinse the mouth to ensure all of the contents have been swallowed.
- Discard any unused portion of the AMRIX capsules after the contents have been sprinkled on applesauce.
-
3 DOSAGE FORMS AND STRENGTHS
Extended-release capsules in the following strengths:
- 15 mg: Capsules are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band on the cap.
- 30 mg: Capsules are blue/red and are embossed in white ink with “30 mg” on the body, and Cephalon “C” logo, “Cephalon,” and a dashed band on the cap.
-
4 CONTRAINDICATIONS
- Hypersensitivity to any component of this product. These adverse reactions may manifest as an anaphylactic reaction, urticaria, facial and/or tongue swelling, or pruritus. Discontinue AMRIX if a hypersensitivity reaction is suspected.
- Concomitant use of monoamine oxidase (MAO) inhibitors or within 14 days after their discontinuation. Hyperpyretic crisis seizures and deaths have occurred in patients receiving cyclobenzaprine (or structurally similar tricyclic antidepressants) concomitantly with MAO inhibitor drugs.
- During the acute recovery phase of myocardial infarction, and in patients with arrhythmias, heart block or conduction disturbances, or congestive heart failure.
- Hyperthyroidism.
-
5 WARNINGS AND PRECAUTIONS
5.1 Serotonin Syndrome
The development of a potentially life-threatening serotonin syndrome has been reported with cyclobenzaprine when used in combination with other drugs, such as selective serotonin reuptake inhibitors (SSRIs), serotonin norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. The concomitant use of AMRIX with MAO inhibitors is contraindicated [see Contraindications (4)]. Serotonin syndrome symptoms may include mental status changes (e.g., confusion, agitation, hallucinations), autonomic instability (e.g., diaphoresis, tachycardia, labile blood pressure, hyperthermia), neuromuscular abnormalities (e.g., tremor, ataxia, hyperreflexia, clonus, muscle rigidity), and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Treatment with AMRIX and any concomitant serotonergic agents should be discontinued immediately if the above reactions occur and supportive symptomatic treatment should be initiated. If concomitant treatment with AMRIX and other serotonergic drugs is clinically warranted, careful observation is advised, particularly during treatment initiation or dose increases.
5.2 Tricyclic Antidepressant-like Effects
Cyclobenzaprine is structurally related to the tricyclic antidepressants, e.g., amitriptyline and imipramine. Tricyclic antidepressants have been reported to produce arrhythmias, sinus tachycardia, prolongation of the conduction time leading to myocardial infarction and stroke [see Contraindications (4)]. AMRIX may enhance the effects of alcohol, barbiturates, and other CNS depressants.
Some of the more serious central nervous system (CNS) reactions noted with the tricyclic antidepressants have occurred in short-term studies of cyclobenzaprine for indications other than muscle spasm associated with acute musculoskeletal conditions, and usually at doses somewhat greater than those recommended for skeletal muscle spasm. If clinically significant CNS symptoms develop, consider discontinuation of AMRIX.
5.3 Use in the Elderly
As a result of a 40% increase in cyclobenzaprine plasma levels and a 56% increase in plasma half-life following administration of AMRIX in elderly subjects as compared to young adults, use of AMRIX is not recommended in the elderly [see Clinical Pharmacology (12.3)].
5.4 Use in Patients with Hepatic Impairment
As a result of two-fold higher cyclobenzaprine plasma levels in subjects with mild hepatic impairment, as compared to healthy subjects, following administration of immediate-release cyclobenzaprine and because there is limited dosing flexibility with AMRIX, use of AMRIX is not recommended in patients with mild, moderate, or severe hepatic impairment [see Clinical Pharmacology (12.3)].
-
6 ADVERSE REACTIONS
The following clinically significant reactions are described in greater detail, in other sections.
- Serotonin Syndrome [see Warnings and Precautions (5.1)]
- Adverse Cardiovascular Effects [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The data described below reflect exposure to AMRIX in 253 patients in 2 clinical trials. AMRIX was studied in two double-blind, parallel-group, placebo-controlled, active-controlled trials of identical design [see Clinical Studies (14)]. The study population was composed of patients with muscle spasms associated with acute painful musculoskeletal conditions. Patients received 15 mg or 30 mg of AMRIX taken orally once daily, cyclobenzaprine immediate-release (IR) 10 mg three times a day, or placebo for 14 days.
The most common adverse reactions (incidence ≥3% in any treatment group and greater than placebo) were dry mouth, dizziness, fatigue, constipation, nausea, dyspepsia, and somnolence (see Table 1).
Table 1: Incidence of the Most Common Adverse Reactions Occurring in ≥ 3% of Patients in any Treatment Group* and Greater Than Placebo in the Two Phase 3, Double-Blind AMRIX Trials
Placebo
AMRIX 15 mg
AMRIX 30 mg
N=128
N=127
N=126
Dry mouth
2%
6%
14%
Dizziness
2%
3%
6%
Fatigue
2%
3%
3%
Constipation
0%
1%
3%
Somnolence
0%
1%
2%
Nausea
1%
3%
3%
Dyspepsia
1%
0%
4%
*AMRIX 15 mg QD, AMRIX 30 mg QD, or cyclobenzaprine IR tablets TID
6.2 Postmarketing Experience
The following adverse reactions have been reported in clinical studies or postmarketing experience with AMRIX, cyclobenzaprine IR, or tricyclic drugs. Because some of these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
In a postmarketing surveillance program of cyclobenzaprine IR, the adverse reactions reported most frequently were drowsiness, dry mouth, and dizziness and adverse reactions reported in 1% to 3% of the patients were: fatigue/tiredness, asthenia, nausea, constipation, dyspepsia, unpleasant taste, blurred vision, headache, nervousness, and confusion.
The following adverse reactions have been reported in postmarketing experience (AMRIX or cyclobenzaprine IR), in clinical studies of cyclobenzaprine IR (incidence <1%), or in postmarketing experience with other tricyclic drugs:
Body as a Whole: Syncope; malaise; chest pain; edema.
Cardiovascular: Tachycardia; arrhythmia; vasodilatation; palpitation; hypotension; hypertension; myocardial infarction; heart block; stroke.
Digestive: Vomiting; anorexia; diarrhea; gastrointestinal pain; gastritis; thirst; flatulence; edema of the tongue; abnormal liver function and rare reports of hepatitis, jaundice, and cholestasis; paralytic ileus, tongue discoloration; stomatitis; parotid swelling.
Endocrine: Inappropriate ADH syndrome.
Hematologic and Lymphatic: Purpura; bone marrow depression; leukopenia; eosinophilia; thrombocytopenia.
Hypersensitivity: Anaphylaxis; angioedema; pruritus; facial edema; urticaria; rash.
Metabolic, Nutritional, and Immune: Elevation and lowering of blood sugar levels; weight gain or loss.
Musculoskeletal: Local weakness; myalgia.
Nervous System and Psychiatric: Seizures, ataxia; vertigo; dysarthria; tremors; hypertonia; convulsions; muscle twitching; disorientation; insomnia; depressed mood; abnormal sensations; anxiety; agitation; psychosis, abnormal thinking and dreaming; hallucinations; excitement; paresthesia; diplopia; serotonin syndrome; neuroleptic malignant syndrome; decreased or increased libido; abnormal gait; delusions; aggressive behavior; paranoia; peripheral neuropathy; Bell’s palsy; alteration in EEG patterns; extrapyramidal symptoms.
Respiratory: Dyspnea.
Skin: Sweating; photosensitization; alopecia.
Special Senses: Ageusia; tinnitus.
Urogenital: Urinary frequency and/or retention; impaired urination; dilatation of urinary tract; impotence; testicular swelling; gynecomastia; breast enlargement; galactorrhea.
-
7 DRUG INTERACTIONS
Based on its structural similarity to tricyclic antidepressants, AMRIX may have life-threatening interactions with MAO inhibitors [see Contraindications (4)], may enhance the effects of alcohol, barbiturates, and other CNS depressants, may enhance the seizure risk in patients taking tramadol, or may block the antihypertensive action of guanethidine and similarly acting compounds.
Postmarketing cases of serotonin syndrome have been reported during combined use of cyclobenzaprine and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors [see Warnings and Precautions (5.1)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Risk Summary
Available data from case reports with AMRIX use in pregnancy have not identified a drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes. In rats, decreased pup body weight and survival was noted at cyclobenzaprine doses ≥10 mg/kg/day (approximately ≥3 times the maximum recommended human dose (MRHD) of 30 mg/day), when administered orally during pregnancy and lactation (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated populations is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the US general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
No adverse embryofetal effects were reported following oral administration of cyclobenzaprine during organogenesis to mice and rabbits at maternal doses up to 20 mg/kg/day (approximately 3 and 15 times the MRHD, respectively, on a mg/m2 basis). Maternal toxicity characterized by decreased body weight gain was observed only in mice at the highest tested dose of 20 mg/kg/day.
Decreased pup body weight and survival were reported in a prenatal and postnatal study where pregnant rats were treated orally with cyclobenzaprine during pregnancy and lactation with maternal doses of 10 and 20 mg/kg/day (approximately 3 and 6 times the MRHD on a mg/m2 basis). Maternal toxicity, characterized by a decreased body weight gain, was observed only at the highest tested dose of 20 mg/kg/day.
8.2 Lactation
Risk Summary
There are no data on the presence of cyclobenzaprine in either human or animal milk, the effects on a breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for AMRIX and any potential adverse effects on the breastfed child from AMRIX or from the underlying maternal condition.
8.5 Geriatric Use
Clinical studies of AMRIX did not include sufficient numbers of patients aged 65 and over to determine the safety and efficacy of AMRIX in the elderly population. The plasma concentration and half-life of cyclobenzaprine are substantially increased in the elderly when compared to the general patient population. Accordingly, use of AMRIX is not recommended in the elderly [see Warnings and Precautions (5.3) and Clinical Pharmacology (12.3)].
8.6 Hepatic Impairment
The use of AMRIX is not recommended in patients with mild, moderate, or severe hepatic impairment [see Warnings and Precautions (5.4) and Clinical Pharmacology (12.3)].
-
9 DRUG ABUSE AND DEPENDENCE
9.3 Dependence
Pharmacologic similarities among the tricyclic drugs require that certain withdrawal symptoms be considered when AMRIX is administered, even though they have not been reported to occur with this drug. Abrupt cessation of treatment after prolonged administration rarely may produce nausea, headache, and malaise. These are not indicative of addiction.
-
10 OVERDOSAGE
10.1 Manifestations
Although rare, deaths may occur from overdosage with AMRIX. Multiple drug ingestion (including alcohol) is common in deliberate cyclobenzaprine overdose. As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment. Signs and symptoms of toxicity may develop rapidly after cyclobenzaprine overdose; therefore, hospital monitoring is required as soon as possible.
The most common effects associated with cyclobenzaprine overdose are drowsiness and tachycardia. Less frequent manifestations include tremor, agitation, coma, ataxia, hypertension, slurred speech, confusion, dizziness, nausea, vomiting, and hallucinations. Rare but potentially critical manifestations of overdose are cardiac arrest, chest pain, cardiac dysrhythmias, severe hypotension, seizures, and neuroleptic malignant syndrome. Changes in the electrocardiogram, particularly in QRS axis or width, are clinically significant indicators of cyclobenzaprine toxicity. Other potential effects of overdosage include any of the symptoms listed under Adverse Reactions (6).
10.2 Management
General
As management of overdose is complex and changing, it is recommended that the physician contact a poison control center for current information on treatment.
In order to protect against the rare but potentially critical manifestations described above, obtain an ECG and immediately initiate cardiac monitoring. Protect the patient’s airway, establish an intravenous line, and initiate gastric decontamination. Observation with cardiac monitoring and observation for signs of CNS or respiratory depression, hypotension, cardiac dysrhythmias and/or conduction blocks, and seizures is necessary. If signs of toxicity occur at any time during this period, extended monitoring is required. Monitoring of plasma drug levels should not guide management of the patient. Dialysis is probably of no value because of low plasma concentrations of the drug.
Gastrointestinal Decontamination
All patients suspected of an overdose with AMRIX should receive gastrointestinal decontamination. This should include large volume gastric lavage followed by activated charcoal. If consciousness is impaired, the airway should be secured prior to lavage and emesis is contraindicated.
Cardiovascular
A maximal limb-lead QRS duration of 0.10 seconds may be the best indication of the severity of the overdose. Serum alkalinization, to a pH of 7.45 to 7.55, using intravenous sodium bicarbonate and hyperventilation (as needed), should be instituted for patients with dysrhythmias and/or QRS widening. A pH >7.60 or a pCO2 <20 mmHg is undesirable. Dysrhythmias unresponsive to sodium bicarbonate therapy/hyperventilation may respond to lidocaine, bretylium, or phenytoin. Type 1A and 1C antiarrhythmics are generally contraindicated (e.g., quinidine, disopyramide, and procainamide).
CNS
In patients with CNS depression, early intubation is advised because of the potential for abrupt deterioration. Seizures should be controlled with benzodiazepines or, if these are ineffective, other anticonvulsants (e.g., phenobarbital, phenytoin). Physostigmine is not recommended except to treat life-threatening symptoms that have been unresponsive to other therapies, and then only in close consultation with a poison control center.
Psychiatric Follow-Up
Since overdosage is often deliberate, patients may attempt suicide by other means during the recovery phase. Psychiatric referral may be appropriate.
Pediatric Management
The principles of management of child and adult overdosage are similar. It is strongly recommended that the physician contact the local poison control center for specific pediatric treatment.
-
11 DESCRIPTION
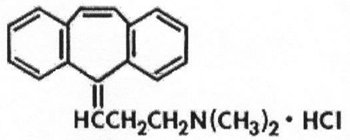
AMRIX is a skeletal muscle relaxant which relieves muscle spasm of local origin without interfering with muscle function. The active ingredient in AMRIX extended-release capsules is cyclobenzaprine hydrochloride, USP. Cyclobenzaprine hydrochloride (HCl) is a white, crystalline tricyclic amine salt with the empirical formula C20H21N·HCl and a molecular weight of 311.9. It has a melting point of 217°C, and a pKa of 8.47 at 25°C. It is freely soluble in water and alcohol, sparingly soluble in isopropanol, and insoluble in hydrocarbon solvents. If aqueous solutions are made alkaline, the free base separates. Cyclobenzaprine HCl is designated chemically as 3-(5H-dibenzo[a,d] cyclohepten-5-ylidene)-N,N-dimethyl-1-propanamine hydrochloride, and has the following structural formula:
AMRIX extended-release capsules for oral administration are supplied in 15 and 30 mg strengths. AMRIX capsules contain the following inactive ingredients: diethyl phthalate NF, ethylcellulose NF (Ethocel Standard 10 Premium), gelatin, Opadry® Clear YS-1-7006, sugar spheres NF (20-25 mesh), and titanium dioxide. AMRIX 15 mg capsules also contain D&C yellow #10, FD&C green #3, and FD&C red #40. AMRIX 30 mg capsules also contain FD&C blue #1, FD&C blue #2, FD&C red #40, and FD&C yellow #6.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Cyclobenzaprine relieves skeletal muscle spasm of local origin without interfering with muscle function. Cyclobenzaprine has not been shown to be effective in muscle spasm due to central nervous system disease. In animal models, cyclobenzaprine reduced or abolished skeletal muscle hyperactivity. Animal studies indicate that cyclobenzaprine does not act at the neuromuscular junction or directly on skeletal muscle. Such studies show that cyclobenzaprine acts primarily within the central nervous system at the brain stem as opposed to the spinal cord level, although an overlapping action on the latter may contribute to its overall skeletal muscle relaxant activity. Evidence suggests that the net effect of cyclobenzaprine is a reduction of tonic somatic motor activity, influencing both gamma (γ) and alpha (α) motor systems. Pharmacological studies in animals demonstrated a similarity between the effects of cyclobenzaprine and the structurally related tricyclic antidepressants, including reserpine antagonism, norepinephrine potentiation, potent peripheral and central anticholinergic effects, and sedation. Cyclobenzaprine caused slight to moderate increase in heart rate in animals.
12.3 Pharmacokinetics
Absorption
Following single-dose administration of AMRIX 15 mg and 30 mg in healthy adult subjects (n=15), Cmax, AUC0-168h and AUC0-∞ increased in an approximately dose-proportional manner from 15 mg to 30 mg. The time to peak plasma cyclobenzaprine concentration (Tmax) was 7 to 8 hours for both doses of AMRIX.
A food effect study conducted in healthy adult subjects (n=15) utilizing a single dose of AMRIX 30 mg demonstrated a statistically significant increase in bioavailability when AMRIX 30 mg was given with food relative to the fasted state. There was a 35% increase in peak plasma cyclobenzaprine concentration (Cmax) and a 20% increase in exposure (AUC0-168h and AUC0-∞) in the presence of food. No effect, however, was noted in Tmax or the shape of the mean plasma cyclobenzaprine concentration versus time profile. Cyclobenzaprine in plasma was first detectable in both the fed and fasted states at 1.5 hours.
When the contents of AMRIX capsules were administered by sprinkling on applesauce, it was found to be bioequivalent to the same dose when administered as an intact capsule.
In a multiple-dose study utilizing AMRIX 30 mg administered once daily for 7 days in a group of healthy adult subjects (n=35), a 2.5-fold accumulation of plasma cyclobenzaprine levels was noted at steady-state.
Metabolism and Excretion
Cyclobenzaprine is extensively metabolized and is excreted primarily as glucuronides via the kidney. Cytochromes P-450 3A4, 1A2, and, to a lesser extent, 2D6, mediate N-demethylation, one of the oxidative pathways for cyclobenzaprine. Cyclobenzaprine has an elimination half-life of 32 hours (range 8-37 hours; n=18); plasma clearance is 0.7 L/min following single-dose administration of AMRIX.
Special Populations
Elderly
Although there were no notable differences in Cmax or Tmax, cyclobenzaprine plasma AUC is increased by 40% and the plasma half-life of cyclobenzaprine is prolonged in elderly subjects greater than 65 years of age (50 hours) after dosing with AMRIX compared to younger subjects 18 to 45 years of age (32 hours). Pharmacokinetic characteristics of cyclobenzaprine following multiple-dose administration of AMRIX in the elderly were not evaluated.
Hepatic Impairment
In a pharmacokinetic study of immediate-release cyclobenzaprine in 16 subjects with hepatic impairment (15 mild, 1 moderate per Child-Pugh score), both AUC and Cmax were approximately double the values seen in the healthy control group. The pharmacokinetics of cyclobenzaprine in subjects with severe hepatic impairment is not known.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies were conducted in CD-1 mice and Sprague-Dawley rats with oral cyclobenzaprine to evaluate its carcinogenic potential. In an 81-week carcinogenicity study, metastatic hemangiosarcoma was seen in 3 of 21 male mice at 10 mg/kg/day (approximately 2 times the maximum recommended human dose (MRHD) of 30 mg/day on a mg/m2 basis). In a 105-week carcinogenicity study, malignant astrocytoma was seen in 3 of 50 male rats at 10 mg/kg/day (approximately 3 times the MRHD on a mg/m2 basis). There were no tumor findings in female mice or rats.
Mutagenesis
Cyclobenzaprine HCl was not mutagenic or clastogenic in the following assays: an in vitro Ames bacterial mutation assay, in vitro Chinese hamster ovary (CHO) cell chromosomal aberration test, and in vivo mouse bone marrow micronucleus assay.
Impairment of Fertility
Cyclobenzaprine HCl, when administered 70 and 14 days prior to mating to male and female rats, respectively, had no effects on fertility or reproductive performance at oral doses up to 20 mg/kg/day (approximately 6.5 times the MRHD on a mg/m2 basis).
13.2 Animal Toxicology and/or Pharmacology
In a 67-week study with rats that received cyclobenzaprine at oral doses of 10, 20, or 40 mg/kg/day (3 to 15 times the MRHD on mg/m2 basis), there were findings in the liver consisting of midzonal vacuolation with lipidosis for males and midzonal and centrilobular hepatocytic enlargement for females. In addition, there were findings of centrilobular coagulative necrosis. In the higher dose groups, these microscopic changes were seen after 26 weeks and even earlier in rats that died prior to 26 weeks; at lower doses, these changes were not seen until after 26 weeks.
In a 26-week study with Cynomolgus monkeys that received cyclobenzaprine at oral of doses of 2.5, 5, 10, or 20 mg/kg/day, one monkey at 20 mg/kg/day (15 times the MRHD on mg/m2 basis) was euthanized in week 17. Morbidity for this animal was attributed to findings of chronic pancreatitis, cholecystitis, cholangitis, and focal liver necrosis.
-
14 CLINICAL STUDIES
Efficacy was assessed in two double-blind, parallel-group, active-controlled, placebo-controlled studies of identical design of AMRIX 15 mg and 30 mg taken once daily, between 6:00 and 7:00 PM, cyclobenzaprine 10 mg three times a day, or placebo for 14 days in patients with muscle spasms associated with acute painful musculoskeletal conditions.
There were significant differences in the primary efficacy analysis, the patient’s rating of medication helpfulness, between the AMRIX 15 mg group and the placebo group at Days 4 and 14 in one study and between the AMRIX 30 mg group and the placebo group at Day 4 in the second study.
Table 2: Patients’ Rating of Medication Helpfulness - Study 1*
Day 4
Day 14
Number of Patients (%)
Number of Patients (%)
Placebo
(N = 64)
AMRIX 30 mg
(N = 64)
Placebo
(N = 64)
AMRIX 30 mg
(N = 64)
Excellent
1 (2%)
3 (5%)
12 (19%)
15 (23%)
Very Good
5 (8%)
13 (20%)
9 (14%)
19 (30%)
Good
15 (23%)
22 (34%)
10 (16%)
15 (23%)
Fair
24 (38%)
20 (31%)
16 (25%)
10 (16%)
Poor
10 (16%)
5 (8%)
9 (14%)
4 (6%)
Missing
9 (14%)
1 (2%)
8 (13%)
1 (2%)
* Percentages are rounded to the nearest whole percent.
Table 3: Patients’ Rating of Medication Helpfulness - Study 2*
Day 4
Day 14
Number of Patients (%)
Number of Patients (%)
Placebo
(N = 64)
AMRIX 15 mg
(N = 63)
Placebo
(N = 64)
AMRIX 15 mg
(N = 63)
Excellent
1 (2%)
2 (3%)
10 (16%)
13 (21%)
Very Good
10 (16%)
12 (19%)
12 (19%)
21 (33%)
Good
14 (22%)
21 (33%)
13 (20%)
9 (14%)
Fair
16 (25%)
17 (27%)
14 (22%)
10 (16%)
Poor
19 (30%)
6 (10%)
12 (19%)
5 (8%)
Missing
4 (6%)
5 (8%)
3 (5%)
5 (8%)
* Percentages are rounded to the nearest whole percent.
In addition, one of the two studies demonstrated significant differences between the AMRIX 30 mg group and the placebo group in terms of patient-rated relief from local pain due to muscle spasm at Day 4 and Day 8, in patient-rated restriction of movement at Day 4 and Day 8, and in patient-rated global impression of change at Day 4, Day 8, and Day 14.
In both studies, there were no significant treatment differences between the AMRIX treatment groups and the placebo group in physician's global assessment, patient-rated restriction in activities of daily living, or quality of nighttime sleep.
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
AMRIX extended-release capsules are available in 15 and 30 mg strengths, packaged in bottles of 60 capsules. AMRIX 15 mg capsules (NDC: 63459-700-60) are orange/orange and are embossed in blue ink with “15 mg” on the body, and Cephalon “C” logo, “Cephalon”, and a dashed band on the cap. AMRIX 30 mg capsules (NDC: 63459-701-60) are blue/red and are embossed in white ink with “30 mg” on the body, and Cephalon “C” logo, “Cephalon”, and a dashed band on the cap.
-
17 PATIENT COUNSELING INFORMATION
See FDA-approved patient labeling (Patient Information).
- Instruct patients to swallow AMRIX capsules intact or to sprinkle capsule contents on a tablespoon of applesauce and swallow immediately without chewing.
- Advise patients to stop taking AMRIX and to notify their physician right away if they experience symptoms of an allergic reaction, such as difficulty breathing, hives, swelling of face or tongue, or itching.
- Advise patients that AMRIX should not be taken with MAO inhibitors or within 14 days after their discontinuation.
- Caution patients about the risk of serotonin syndrome with concomitant use of AMRIX and other drugs, such as SSRIs, SNRIs, TCAs, tramadol, bupropion, meperidine, verapamil, or MAO inhibitors. Advise patients of the signs and symptoms of serotonin syndrome [see Warnings and Precautions (5.1)] and instruct patients to seek medical care immediately if they experience these symptoms.
- Advise patients to stop taking AMRIX and to notify their physician right away if they experience arrhythmias or tachycardia.
- Advise patients that AMRIX may enhance the impairment effects of alcohol. These effects may also be seen if AMRIX is taken with other CNS depressants.
- Caution patients about operating an automobile or other hazardous machinery until it is reasonably certain that AMRIX therapy will not adversely affect their ability to engage in such activities.
- Advise patients to take AMRIX at approximately the same time each day.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Manufactured By:
Adare Pharmaceuticals, Inc.
Vandalia, OH 45377AMR-010
Rev. 4/2019
US Patent Nos. 7387793, 7544372, 7790199, 7820203, 7829121, 9375410, 9399025
© 2004-2019 Teva Pharmaceuticals USA, Inc.
All rights reserved.
-
Patient Information
PATIENT INFORMATION
AMRIX® (am-rix)
(cyclobenzaprine hydrochloride extended-release capsules)What is AMRIX? AMRIX is a prescription medicine used along with rest and physical therapy to help treat muscle spasm due to acute, painful musculoskeletal problems.
AMRIX should only be used for up to 2 or 3 weeks. It is not known if AMRIX is effective when used for longer periods.
It is not known if AMRIX is safe and effective in children.Do not take AMRIX if you:
- are allergic to cyclobenzaprine or any of the ingredients in AMRIX. See the end of this Patient Information leaflet for a complete list of ingredients in AMRIX.
Talk to your healthcare provider or get medical help right away if you have symptoms of an allergic reaction such as:
- difficulty breathing
- hives
- swelling of your face or tongue
- itching
- are taking certain antidepressants, known as monoamine oxidase (MAO) inhibitors or it has been 14 days or less since you stopped taking a MAO inhibitor. Ask your healthcare provider or pharmacist for a list of these medicines if you are not sure.
- have had a recent heart attack
- have heart rhythm problems (arrhythmias)
- have heart failure
- have an overactive thyroid (hyperthyroidism)
Before taking AMRIX, tell your healthcare provider about all of your medical conditions, including if you:
- have a history of eye problems including glaucoma
- have heart problems or have had a heart attack
- have liver problems
- have trouble emptying your bladder (urinary retention)
- are pregnant or plan to become pregnant. It is not known if AMRIX will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if AMRIX passes into your breast milk. Talk to your healthcare provider about the best way to best way to feed your baby if you take AMRIX.
Tell your healthcare provider about all the medicines you take including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Especially tell your healthcare provider if you take:
- a medicine to treat depression, mood, anxiety, psychotic, or thought disorders
- a pain medicine called tramadol or meperidine
- barbiturates or other medicines that depress your central nervous system (CNS depressants)
- a medicine that prevents nerve impulses (anticholinergic medicines)
- a medicine to help quit smoking called bupropion
- a blood pressure medicine called verapamil
Know the medicines you take. Keep a list of your medicines and show it to your healthcare provider or pharmacist when you get a new medicine.
How should I take AMRIX?
- Take AMRIX exactly as your healthcare provider tells you to take it.
- Your healthcare provider will tell you how much AMRIX to take and when to take it.
- Your healthcare provider may change your AMRIX dose if needed.
- Take AMRIX around the same time every day.
- Swallow AMRIX capsules whole.
- If you have difficulty swallowing AMRIX capsules, tell your healthcare provider. Your healthcare provider may recommend opening the AMRIX capsule and mixing the contents with applesauce.
- AMRIX should only be taken for short periods (up to two or three weeks).
- If you take too much AMRIX, call your healthcare provider or go to the nearest hospital emergency room right away.
What should I avoid while taking AMRIX?
You should not drink alcohol until you know how AMRIX affects you. Taking AMRIX with alcohol or other medicines that depress your central nervous system can slow your thinking and physical response times.
Do not drive, operate machinery, or do other dangerous activities until you know how AMRIX affects you.What are the possible side effects of AMRIX?
AMRIX may cause serious side effects, including:
- Serotonin syndrome is a serious medical condition that may happen when AMRIX is taken with certain other medicines. Call your healthcare provider right away or go to the nearest hospital emergency room if you have some or all of these symptoms suggestive of serotonin syndrome:
- agitation, hallucinations, coma, or other changes in mental status
- coordination problems or muscle twitching (overactive reflexes)
- fast heartbeat, high or low blood pressure
- sweating or fever
- nausea, vomiting, or diarrhea
- muscle stiffness or tightness
- AMRIX may cause serious side effects that may lead to heart attack or stroke. Call your healthcare provider right away or go to the nearest hospital emergency room if you have:
- irregular or abnormal heartbeats (arrhythmias)
- fast heartbeat (tachycardia)
The most common side effects of AMRIX include:
- dry mouth
- dizziness
- fatigue
- constipation
- nausea
- upset stomach
- drowsiness
These are not all the possible side effects of AMRIX.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store AMRIX?
- Store AMRIX at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep AMRIX in a tightly closed container, and keep AMRIX out of light.
- Keep AMRIX and all medicines out of the reach of children.
General information about the safe and effective use of AMRIX.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use AMRIX for a condition for which it was not prescribed. Do not give AMRIX to other people, even if they have the same symptoms you have. It may harm them.
You can ask your pharmacist or healthcare provider for information about AMRIX that is written for healthcare professionals.What are the ingredients in AMRIX?
Active Ingredient: cyclobenzaprine hydrochloride USP
Inactive Ingredients: diethyl phthalate NF, ethylcellulose NF (Ethocel Standard 10 Premium), gelatin, Opadry® Clear YS-1-7006, sugar spheres NF (20-25 mesh), and titanium dioxide.
AMRIX 15 mg capsules also contain: D&C yellow #10, FD&C green #3, and FD&C red #40.
AMRIX 30 mg capsules also contain: FD&C blue #1, FD&C blue #2, FD&C red #40, and FD&C yellow #6.
Distributed By:
Teva Pharmaceuticals USA, Inc.
North Wales, PA 19454Manufactured By:
Adare Pharmaceuticals, Inc.
Vandalia, OH 45377AMRPL-005
For more information, go to www.AMRIX.com or call 1-888-483-8279.
© 2004-2019 Teva Pharmaceuticals USA, Inc.
All rights reserved.This Patient Information has been approved by the U.S. Food and Drug Administration Revised: April 2019
- are allergic to cyclobenzaprine or any of the ingredients in AMRIX. See the end of this Patient Information leaflet for a complete list of ingredients in AMRIX.
-
INSTRUCTIONS FOR USE
INSTRUCTIONS FOR USE
AMRIX® (am-rix)
(cyclobenzaprine hydrochloride extended-release capsules)
Read this Instructions for Use before you prepare your first dose of AMRIX mixed with applesauce using the capsule sprinkle method, each time you get a refill, and as needed. There may be new information. Ask your healthcare provider or pharmacist if you have any questions about how to mix or give a dose of AMRIX using the capsule sprinkle method.
Important Information:
- Do not chew AMRIX capsules or the granules that are in the capsules.
- The capsule sprinkle method for mixing the contents of AMRIX with applesauce may be used for adults who cannot swallow capsules. Do not use any other food in the place of applesauce.
Preparing a dose of AMRIX using the capsule sprinkle method.
Before you prepare a dose of AMRIX mixed with applesauce using the capsule sprinkle method, gather the following supplies:
- paper towels
- tablespoon
- applesauce
- cup of water
Step 1: Choose a clean, flat work surface. Place a clean paper towel on the work surface. Then place the other supplies on the paper towel. Step 2: Wash and dry your hands well. Step 3: Check the dose that was prescribed by your healthcare provider. Take out the number of AMRIX capsules needed to prepare your dose. Place them on the paper towel. Step 4: Place enough applesauce to fill your tablespoon. Set the tablespoon down on the paper towel. 
Step 5: Hold the AMRIX capsule in an upright position (vertical) directly over the tablespoon. Hold each end of the AMRIX capsule between your thumbs and index (pointer) fingers. 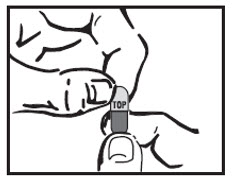
Step 6: Carefully twist both ends of the AMRIX capsule in opposite directions to open it. Be careful not to spill the capsule contents. 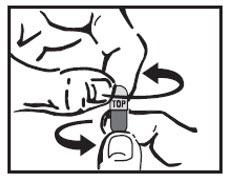
Step 7: Sprinkle the contents of the AMRIX capsule onto the applesauce.
- Check the capsule shells to make sure they are empty.
- Throw away the empty capsule shells.
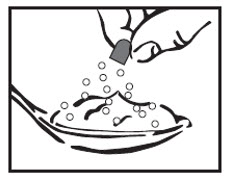
Step 8: Pick up the tablespoon and swallow the AMRIX capsule contents and applesauce mixture right away. Do not chew the AMRIX capsule contents and applesauce mixture. 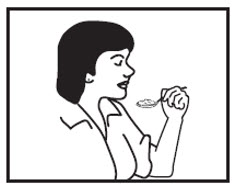
Step 9: Rinse your mouth with a sip of water and swallow to make sure that all of the AMRIX granules have been swallowed. 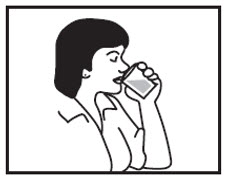
Step 10: Throw away any unused AMRIX capsule content and applesauce mixture. Do not keep any AMRIX capsule content and applesauce mixture for future use. How should I store AMRIX?
- Store AMRIX capsules at room temperature between 68°F to 77°F (20°C to 25°C).
Keep AMRIX capsules and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
AMRIFU-001
Issued: April 2019
- Package/Label Display Panel
- Package/Label Display Panel
-
INGREDIENTS AND APPEARANCE
AMRIX
cyclobenzaprine hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63459-700 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOBENZAPRINE HYDROCHLORIDE (UNII: 0VE05JYS2P) (CYCLOBENZAPRINE - UNII:69O5WQQ5TI) CYCLOBENZAPRINE HYDROCHLORIDE 15 mg Inactive Ingredients Ingredient Name Strength DIETHYL PHTHALATE (UNII: UF064M00AF) ETHYLCELLULOSE (10 MPA.S) (UNII: 3DYK7UYZ62) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C GREEN NO. 3 (UNII: 3P3ONR6O1S) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color ORANGE Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 15;mg;C;Cephalon Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63459-700-22 5 in 1 CARTON 10/02/2007 10/31/2018 1 NDC: 63459-700-02 2 in 1 BLISTER PACK; Type 0: Not a Combination Product 2 NDC: 63459-700-15 10 in 1 CARTON 10/02/2007 05/31/2015 2 NDC: 63459-700-11 1 in 1 BLISTER PACK; Type 0: Not a Combination Product 3 NDC: 63459-700-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 10/02/2007 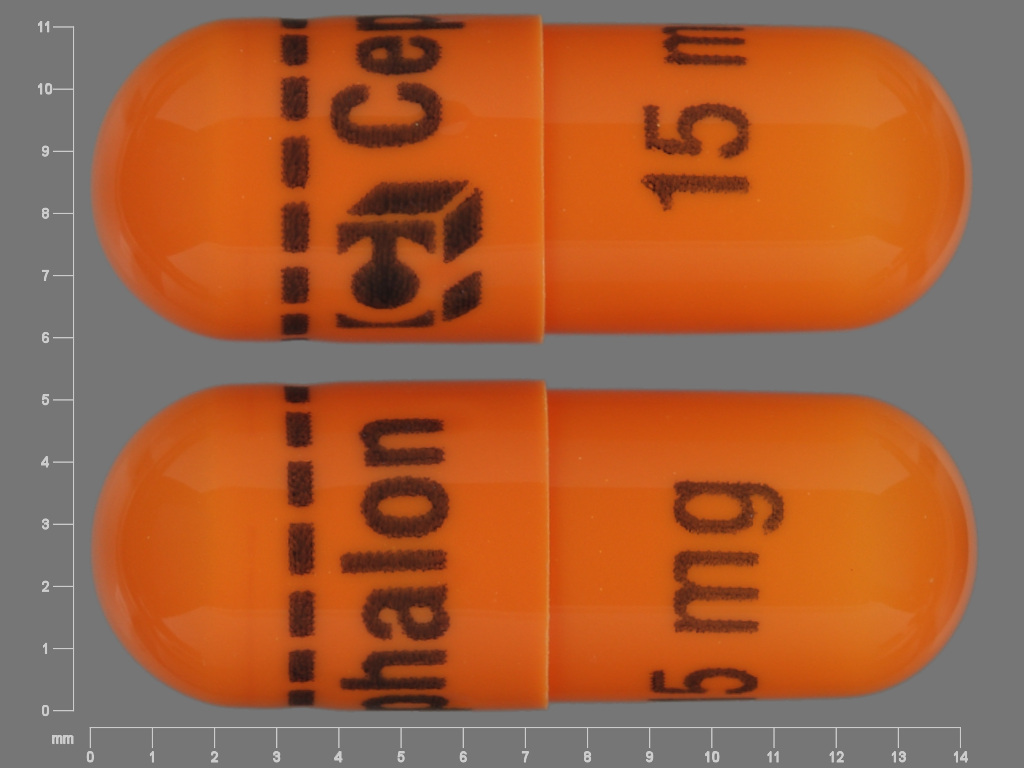
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021777 10/02/2007 AMRIX
cyclobenzaprine hydrochloride capsule, extended releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 63459-701 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYCLOBENZAPRINE HYDROCHLORIDE (UNII: 0VE05JYS2P) (CYCLOBENZAPRINE - UNII:69O5WQQ5TI) CYCLOBENZAPRINE HYDROCHLORIDE 30 mg Inactive Ingredients Ingredient Name Strength DIETHYL PHTHALATE (UNII: UF064M00AF) ETHYLCELLULOSE (10 MPA.S) (UNII: 3DYK7UYZ62) GELATIN (UNII: 2G86QN327L) HYPROMELLOSE 2910 (6 MPA.S) (UNII: 0WZ8WG20P6) POLYETHYLENE GLYCOL 400 (UNII: B697894SGQ) POLYETHYLENE GLYCOL 8000 (UNII: Q662QK8M3B) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C BLUE NO. 2 (UNII: L06K8R7DQK) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color BLUE, RED Score no score Shape CAPSULE Size 14mm Flavor Imprint Code 30;mg;C;Cephalon Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 63459-701-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 11/01/2007 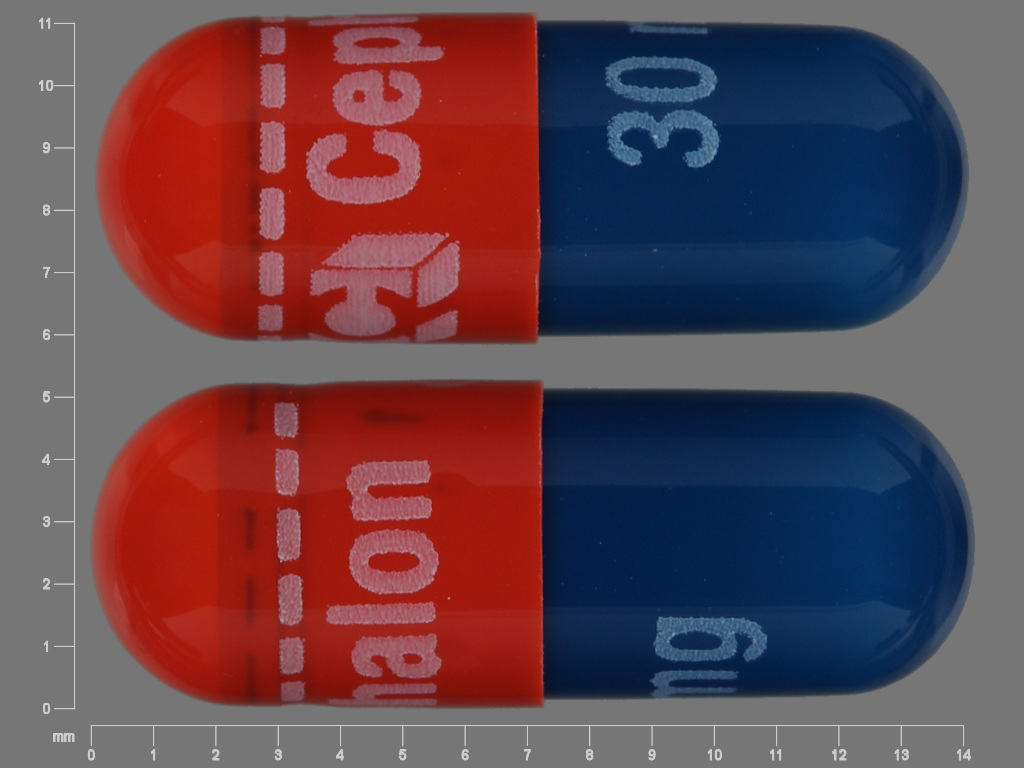
Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA021777 10/01/2007 Labeler - Cephalon, Inc. (183236314)
Trademark Results [AMRIX]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 AMRIX 78261264 3353178 Live/Registered |
TEVA PHARMACEUTICALS INTERNATIONAL GMBH 2003-06-11 |
 AMRIX 77307173 3684884 Live/Registered |
TEVA PHARMACEUTICALS INTERNATIONAL GMBH 2007-10-18 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.

