TUSSIN DM- dextromethorphan hydrobromide and guaifenesin liquid
Tussin DM by
Drug Labeling and Warnings
Tussin DM by is a Otc medication manufactured, distributed, or labeled by davAgen Pharmaceutical, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- ACTIVE INGREDIENT
- Uses
-
Warnings
Do not use
- if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have cough that occurs with too much phlegm (mucus) or a cough that lasts or is chronic such as occurs with smoking, asthma, chronic bronchitis or emphysema
- Other information
- Inactive ingredients
- Directions
- SPL UNCLASSIFIED SECTION
-
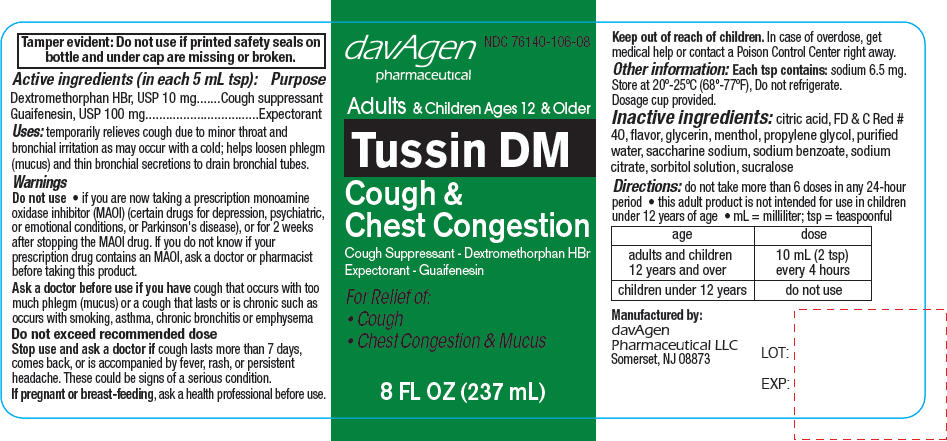
PRINCIPAL DISPLAY PANEL - 237 mL Bottle Label
davAgen
pharmaceuticalNDC: 76140-106-08
Adults & Children Ages 12 & Older
Tussin DM
Cough &
Chest CongestionCough Suppressant - Dextromethorphan HBr
Expectorant - GuaifenesinFor Relief of:
- Cough
- Chest Congestion & Mucus
8 FL OZ (237 mL)
-
INGREDIENTS AND APPEARANCE
TUSSIN DM
dextromethorphan hydrobromide and guaifenesin liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 76140-106 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Dextromethorphan Hydrobromide (UNII: 9D2RTI9KYH) (Dextromethorphan - UNII:7355X3ROTS) Dextromethorphan Hydrobromide 10 mg in 5 mL Guaifenesin (UNII: 495W7451VQ) (Guaifenesin - UNII:495W7451VQ) Guaifenesin 100 mg in 5 mL Inactive Ingredients Ingredient Name Strength Sodium Benzoate (UNII: OJ245FE5EU) Citric Acid Monohydrate (UNII: 2968PHW8QP) Sorbitol (UNII: 506T60A25R) Saccharin Sodium (UNII: SB8ZUX40TY) Sucralose (UNII: 96K6UQ3ZD4) FD&C Red No. 40 (UNII: WZB9127XOA) Propylene Glycol (UNII: 6DC9Q167V3) Glycerin (UNII: PDC6A3C0OX) Sodium Citrate (UNII: 1Q73Q2JULR) Menthol (UNII: L7T10EIP3A) Water (UNII: 059QF0KO0R) Product Characteristics Color RED Score Shape Size Flavor TROPICAL FRUIT PUNCH, ORANGE, MENTHOL Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 76140-106-08 1 in 1 CARTON 1 236 mL in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH FINAL part341 04/10/2013 Labeler - davAgen Pharmaceutical, LLC (967545935) Establishment Name Address ID/FEI Business Operations davAgen Pharmaceutical, LLC 967545935 MANUFACTURE(76140-106) , PACK(76140-106) , LABEL(76140-106) , ANALYSIS(76140-106)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.