Adapalene by YYBA CORP / Taro Pharmaceuticals U.S.A., Inc. / Taro Pharmaceuticals Inc. ADAPALENE gel
Adapalene by
Drug Labeling and Warnings
Adapalene by is a Otc medication manufactured, distributed, or labeled by YYBA CORP, Taro Pharmaceuticals U.S.A., Inc., Taro Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
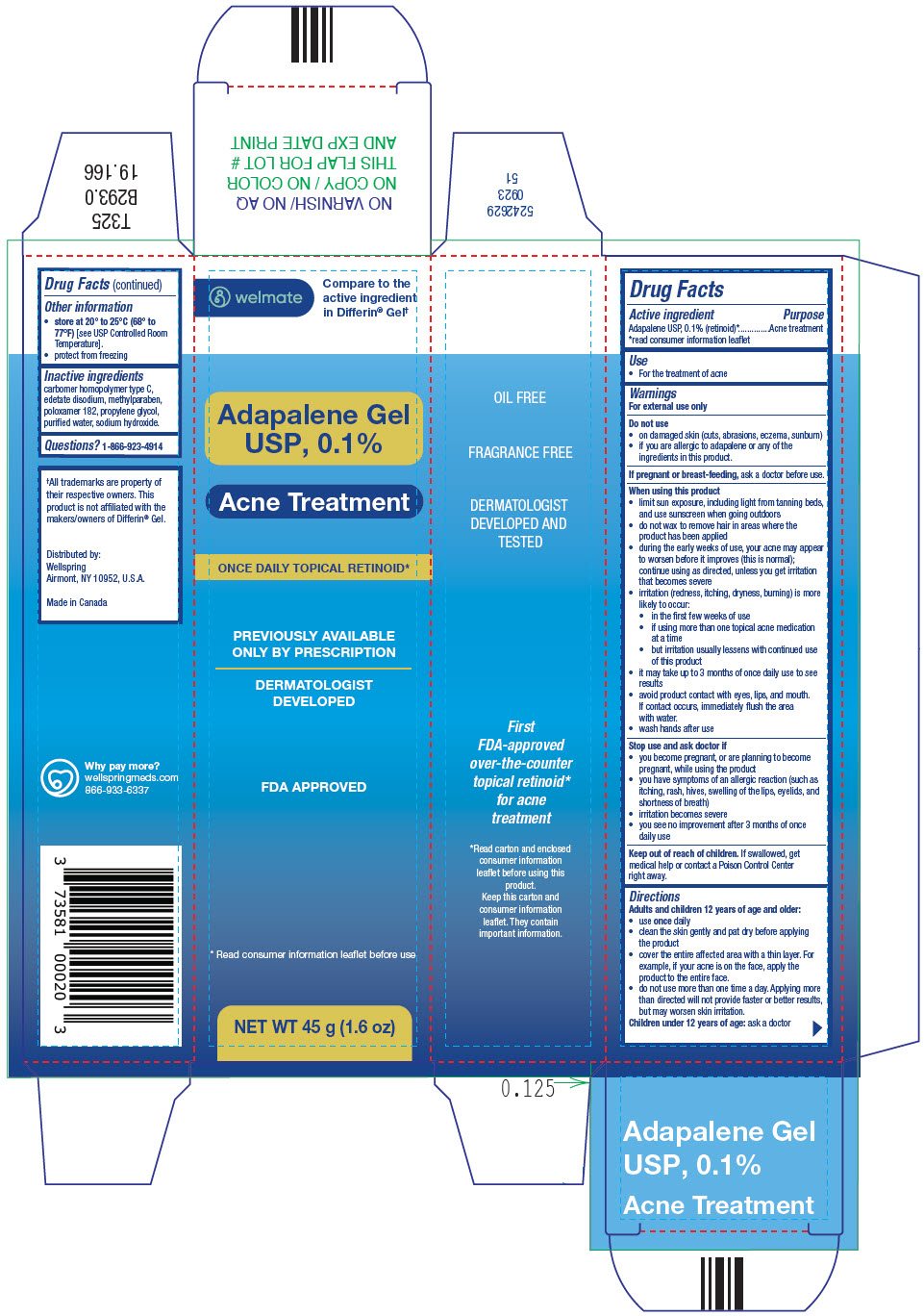
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Use
-
Warnings
For external use only
Do not use
- on damaged skin (cuts, abrasions, eczema, sunburn)
- if you are allergic to adapalene or any of the ingredients in this product.
When using this product
- limit sun exposure, including light from tanning beds, and use sunscreen when going outdoors
- do not wax to remove hair in areas where the product has been applied
- during the early weeks of use, your acne may appear to worsen before it improves (this is normal); continue using as directed, unless you get irritation that becomes severe
- irritation (redness, itching, dryness, burning) is more likely to occur:
- in the first few weeks of use
- if using more than one topical acne medication at a time
- but irritation usually lessens with continued use of this product
- it may take up to 3 months of once daily use to see results
- avoid product contact with eyes, lips, and mouth. If contact occurs, immediately flush the area with water.
- wash hands after use
Stop use and ask doctor if
- you become pregnant, or are planning to become pregnant, while using the product
- you have symptoms of an allergic reaction (such as itching, rash, hives, swelling of the lips, eyelids, and shortness of breath)
- irritation becomes severe
- you see no improvement after 3 months of once daily use
-
Directions
Adults and children 12 years of age and older:
- use oncedaily
- clean the skin gently and pat dry before applying the product
- cover the entire affected area with a thin layer. For example, if your acne is on the face, apply the product to the entire face.
- do not use more than one time a day. Applying more than directed will not provide faster or better results, but may worsen skin irritation.
Children under 12 years of age:ask a doctor
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 45 g Tube Carton
-
INGREDIENTS AND APPEARANCE
ADAPALENE
adapalene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 73581-214 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ADAPALENE (UNII: 1L4806J2QF) (ADAPALENE - UNII:1L4806J2QF) ADAPALENE 1 mg in 1 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE C (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 4Q93RCW27E) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLPARABEN (UNII: A2I8C7HI9T) POLOXAMER 182 (UNII: JX0HIX6OAG) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) WATER (UNII: 059QF0KO0R) SODIUM HYDROXIDE (UNII: 55X04QC32I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 73581-214-06 1 in 1 CARTON 10/15/2023 1 45 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA215940 10/15/2023 Labeler - YYBA CORP (006339772) Registrant - Taro Pharmaceuticals U.S.A., Inc. (145186370) Establishment Name Address ID/FEI Business Operations Sun Pharma Canada Inc. 243339023 manufacture(73581-214)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.