RIFAMATE- rifampin and isoniazid capsule
Rifamate by
Drug Labeling and Warnings
Rifamate by is a Prescription medication manufactured, distributed, or labeled by sanofi-aventis U.S. LLC, Patheon Pharmaceuticals Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
BOXED WARNING
(What is this?)
WARNING
Severe and sometimes fatal hepatitis associated with isoniazid therapy may occur and may develop even after many months of treatment. The risk of developing hepatitis is age related. Approximate case rates by age are: 0 per 1,000 for persons under 20 years of age, 3 per 1,000 for persons in the 20 to 34 year age group, 12 per 1,000 for persons in the 35 to 49 year age group, 23 per 1,000 for persons in the 50 to 64 year age group, and 8 per 1,000 for persons over 65 years of age. The risk of hepatitis is increased with daily consumption of alcohol. Precise data to provide a fatality rate for isoniazid-related hepatitis is not available; however, in a U.S. Public Health Service Surveillance Study of 13,838 persons taking isoniazid, there were 8 deaths among 174 cases of hepatitis.
Therefore, patients given isoniazid should be carefully monitored and interviewed at monthly intervals. Serum transaminase concentration becomes elevated in about 10% to 20% of patients, usually during the first few months of therapy, but it can occur at any time. Usually enzyme levels return to normal despite continuance of drug, but in some cases progressive liver dysfunction occurs. Patients should be instructed to report immediately any of the prodromal symptoms of hepatitis, such as fatigue, weakness, malaise, anorexia, nausea, or vomiting. If these symptoms appear or if signs suggestive of hepatic damage are detected, isoniazid should be discontinued promptly, since continued use of the drug in these cases has been reported to cause a more severe form of liver damage.
Patients with tuberculosis should be given appropriate treatment with alternative drugs. If isoniazid must be reinstituted, it should be reinstituted only after symptoms and laboratory abnormalities have cleared. The drug should be restarted in very small and gradually increasing doses and should be withdrawn immediately if there is any indication of recurrent liver involvement. Treatment should be deferred in persons with acute hepatic diseases.
-
DESCRIPTION
RIFAMATE is a combination capsule containing 300 mg rifampin and 150 mg isoniazid. The capsules also contain the inactive ingredients: colloidal silicon dioxide, FD&C Blue No. 1, FD&C Red No. 40, gelatin, magnesium stearate, sodium starch glycolate, and titanium dioxide.
Rifampin
Rifampin is a semisynthetic antibiotic derivative of rifamycin SV. Rifampin is a red-brown crystalline powder very slightly soluble in water at neutral pH, freely soluble in chloroform, soluble in ethyl acetate and methanol. Its molecular weight is 822.95 and its chemical formula is C43H58N4O12. The chemical name for rifampin is either:
3-[[(4-methyl-1-piperazinyl)imino]-methyl]-rifamycin
or
5,6,9,17,19,21-hexahydroxy-23-methoxy-2,4,12,16,18,20,22–heptamethyl-8-[N-(4-methyl-1-piperazinyl)formimidoyl]-2,7-(epoxypentadeca[1,11,13]trienimino)naphtho[2,1-b]furan-1,11(2H)-dione 21-acetate.Its structural formula is:
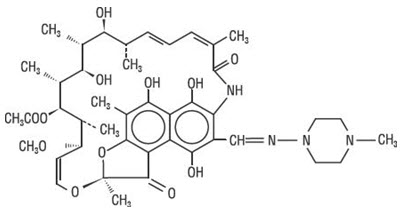
Isoniazid
Isoniazid is the hydrazide of isonicotinic acid. It is a colorless or white crystalline powder or white crystals. It is odorless and slowly affected by exposure to air and light. It is freely soluble in water, sparingly soluble in alcohol and slightly soluble in chloroform and in ether. Its molecular weight is 137.14 and its chemical formula is C6H7N3O.
The chemical name for isoniazid is 4-pyridinecarboxylic acid, hydrazide and its structural formula is:
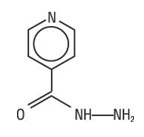
-
CLINICAL PHARMACOLOGY
General
Rifampin
Rifampin is readily absorbed from the gastrointestinal tract. Peak serum levels in healthy adults and pediatric populations vary widely from individual to individual. Following a single 600 mg oral dose of rifampin in healthy adults, the peak serum level averages 7 mcg/mL but may vary from 4 to 32 mcg/mL. Absorption of rifampin is reduced by about 30% when the drug is ingested with food.
In a study of 14 normal human adult males, peak blood levels of rifampin occurred 1½ to 3 hours following oral administration of two RIFAMATE capsules. The peaks ranged from 6.9 to 14 mcg/mL with an average of 10 mcg/mL.
In healthy adults, the biological half-life of rifampin in serum averages 3.35±0.66 hours after a 600 mg oral dose, with increases up to 5.08±2.45 hours reported after a 900 mg dose. With repeated administration, the half-life decreases and reaches average values of approximately 2 to 3 hours. The half-life does not differ in patients with renal failure at doses not exceeding 600 mg daily, and, consequently, no dosage adjustment is required. The half-life of rifampin at a dose of 720 mg daily has not been established in patients with renal failure. Following a single 900 mg oral dose of rifampin in patients with varying degrees of renal insufficiency, the mean half-life increased from 3.6 hours in healthy adults to 5.0, 7.3, and 11.0 hours in patients with glomerular filtration rates of 30 to 50 mL/min, less than 30 mL/min, and in anuric patients, respectively. Refer to the WARNINGS section for information regarding patients with hepatic insufficiency.
After absorption, rifampin is rapidly eliminated in the bile, and an enterohepatic circulation ensues. During this process, rifampin undergoes progressive deacetylation so that nearly all the drug in the bile is in this form in about 6 hours. This metabolite has antibacterial activity. Intestinal reabsorption is reduced by deacetylation, and elimination is facilitated. Up to 30% of a dose is excreted in the urine, with about half as unchanged drug.
Rifampin is widely distributed throughout the body. It is present in effective concentrations in many organs and body fluids, including cerebrospinal fluid. Rifampin is about 80% protein bound. Most of the unbound fraction is not ionized and therefore is diffused freely in tissues.
Pediatrics
In one study, pediatric patients 6 to 58 months old were given rifampin suspended in simple syrup or as dry powder mixed with applesauce at a dose of 10 mg/kg body weight. Peak serum concentrations of 10.7±3.7 and 11.5±5.1 mcg/mL were obtained 1 hour after preprandial ingestion of the drug suspension and the applesauce mixture, respectively. After the administration of either preparation, the t½ of rifampin averaged 2.9 hours. It should be noted that in other studies in pediatric populations, at doses of 10 mg/kg body weight, mean peak serum concentrations of 3.5 mcg/mL to 15 mcg/mL have been reported.
Isoniazid
After oral administration, isoniazid is readily absorbed from the GI tract and produces peak blood levels within 1 to 2 hours which decline to 50% or less within 6 hours. It diffuses readily into all body fluids (cerebrospinal, pleural, and ascitic fluids), tissues, organs, and excreta (saliva, sputum, and feces). Concomitant use with food may reduce the absorption of isoniazid which may reduce RIFAMATE efficacy.
Isoniazid is not substantially bound to plasma proteins. The drug also passes through the placental barrier and into milk in concentrations comparable to those in the plasma. The plasma half-life of isoniazid in patients with normal renal and hepatic function ranges from 1 to 4 hours, depending on the rate of metabolism. From 50% to 70% of a dose of isoniazid is excreted in the urine in 24 hours, mostly as metabolites.
Isoniazid is metabolized in the liver mainly by acetylation and dehydrazination. The rate of acetylation is genetically determined. Approximately 50% of African Americans and Caucasians are "slow inactivators" and the rest are "rapid inactivators"; the majority of Eskimos and Asians are "rapid inactivators."
The rate of acetylation does not significantly alter the effectiveness of isoniazid. However, slow acetylation may lead to higher blood levels of the drug, and thus an increase in toxic reactions.
Pyridoxine (B6) deficiency is sometimes observed in adults with high doses of isoniazid and is probably due to its competition with pyridoxal phosphate for the enzyme apotryptophanase.
Microbiology
Rifampin and isoniazid at therapeutic levels have demonstrated bactericidal activity against both intracellular and extracellular Mycobacterium tuberculosis organisms.
Mechanism of Action
Resistance
Organisms resistant to rifampin are likely to be resistant to other rifamycins. ß-lactamase production should have no effect on rifampin activity.
In the treatment of tuberculosis (see INDICATIONS AND USAGE), the small number of resistant cells present within large populations of susceptible cells can rapidly become predominant. In addition, resistance to rifampin has been determined to occur as single-step mutations of the DNA-dependent RNA polymerase. Since resistance can emerge rapidly, appropriate susceptibility tests should be performed in the event of persistent positive cultures.
-
INDICATIONS AND USAGE
In the treatment of tuberculosis, the small number of resistant cells present within large populations of susceptible cells can rapidly become the predominant type. Since resistance can emerge rapidly, susceptibility tests should be performed in the event of persistent positive cultures during the course of treatment. Bacteriologic smears or cultures should be obtained before the start of therapy to confirm the susceptibility of the organism to rifampin and isoniazid, and they should be repeated throughout therapy to monitor response to the treatment. If test results show resistance to any of the components of RIFAMATE and the patient is not responding to therapy, the drug regimen should be modified.
RIFAMATE is indicated for pulmonary tuberculosis in which organisms are susceptible, and when the patient has been titrated on the individual components and it has therefore been established that this fixed dosage is therapeutically effective.
This fixed-dosage combination drug is not recommended for initial therapy of tuberculosis or for preventive therapy.
A three-drug regimen consisting of rifampin, isoniazid, and pyrazinamide (e.g., RIFATER®) is recommended in the initial phase of short-course therapy which is usually continued for 2 months. The Advisory Council for the Elimination of Tuberculosis, the American Thoracic Society, and Centers for Disease Control and Prevention recommend that either streptomycin or ethambutol be added as a fourth drug in a regimen containing isoniazid (INH), rifampin, and pyrazinamide for initial treatment of tuberculosis unless the likelihood of INH resistance is very low. The need for a fourth drug should be reassessed when the results of susceptibility testing are known. If community rates of INH resistance are currently less than 4%, an initial treatment regimen with less than four drugs may be considered.
Following the initial phase, treatment should be continued with RIFAMATE for at least 4 months. Treatment should be continued for longer if the patient is still sputum or culture positive, if resistant organisms are present, or if the patient is HIV positive.
This drug is not indicated for the treatment of meningococcal infections or asymptomatic carriers of Neisseria meningitidis to eliminate meningococci from the nasopharynx.
-
CONTRAINDICATIONS
RIFAMATE is contraindicated in patients with a history of hypersensitivity to rifampin or isoniazid, or any of the components, or to any of the rifamycins.
Rifampin
Rifampin is contraindicated in patients who are also receiving ritonavir-boosted saquinavir due to an increased risk of severe hepatocellular toxicity. (See PRECAUTIONS, Drug Interactions.)
Rifampin is contraindicated in patients who are also receiving atazanavir, darunavir, fosamprenavir, saquinavir, or tipranavir due to the potential of rifampin to substantially decrease plasma concentrations of these antiviral drugs, which may result in loss of antiviral efficacy and/or development of viral resistance.
Rifampin is contraindicated in patients receiving praziquantel since therapeutically effective blood levels of praziquantel may not be achieved. In patients receiving rifampin who need immediate treatment with praziquantel, alternative agents should be considered. However, if treatment with praziquantel is necessary, rifampin should be discontinued 4 weeks before administration of praziquantel. Treatment with rifampin can then be restarted one day after completion of praziquantel treatment.
-
WARNINGS
RIFAMATE (rifampin and isoniazid capsules USP) is a combination of two drugs, each of which has been associated with liver dysfunction.
Systemic hypersensitivity reactions were reported with both components of RIFAMATE (rifampin and isoniazid). Signs and symptoms of hypersensitivity reactions may include fever, rash, urticaria, angioedema, hypotension, acute bronchospasm, conjunctivitis, thrombocytopenia, neutropenia, elevated liver transaminases or flu-like syndrome (weakness, fatigue, muscle pain, nausea, vomiting, headache, chills, aches, itching, sweats, dizziness, shortness of breath, chest pain, cough, syncope, palpitations). Manifestations of hypersensitivity, such as fever, lymphadenopathy or laboratory abnormalities (including eosinophilia, liver abnormalities) may be present even though rash is not evident. Monitor patients receiving RIFAMATE for signs and/or symptoms of hypersensitivity reactions. If these signs or symptoms occur, discontinue RIFAMATE and administer supportive measures.
Cases of severe cutaneous adverse reactions such as drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome have been reported with both components of RIFAMATE (rifampin and isoniazid). If symptoms or signs of severe cutaneous adverse reactions develop, discontinue RIFAMATE immediately and institute appropriate therapy.
Rifampin
Hepatotoxicity of hepatocellular, cholestatic, and mixed patterns has been reported in patients treated with rifampin. Severity ranged from asymptomatic elevations in liver enzymes, isolated jaundice/hyperbilirubinemia, symptomatic self-limited hepatitis to fulminant liver failure and death. Severe hepatic dysfunction including fatalities were reported in patients with liver disease and in patients taking rifampin with other hepatotoxic agents.
Monitor for symptoms and clinical/laboratory signs of liver injury, especially if treatment is prolonged or given with other hepatotoxic drugs. Patients with impaired liver function should be given rifampin only in cases of necessity and then under strict medical supervision. In these patients, careful monitoring of liver function should be done prior to therapy and then every 2 to 4 weeks during therapy. If signs of hepatic damage occur or worsen, discontinue RIFAMATE.
Rifampin has enzyme-inducing properties, including induction of delta amino levulinic acid synthetase. Isolated reports have associated porphyria exacerbation with rifampin administration.
Cases of severe cutaneous adverse reactions (SCAR) such as Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), acute generalized exanthematous pustulosis (AGEP), and drug reaction with eosinophilia and systemic symptoms (DRESS) syndrome have been reported with rifampin. If symptoms or signs of severe cutaneous adverse reactions develop, discontinue RIFAMATE immediately and institute appropriate therapy.
Rifampin may cause vitamin K–dependent coagulation disorders and bleeding (see ADVERSE REACTIONS). Monitor coagulation tests during rifampin treatment (prothrombin time and other coagulation tests) in patients at risk of vitamin K deficiency (such as those with chronic liver disease, poor nutritional status, on prolonged antibacterial drugs or anticoagulants). Consider discontinuation of RIFADIN if abnormal coagulation tests and/or bleeding occur. Supplemental vitamin K administration should be considered when appropriate.
Postmarketing reports suggest that concomitant administration of high doses of cefazolin and rifampin may prolong the prothrombin time, leading to severe vitamin K–dependent coagulation disorders that may be life-threatening or fatal. Avoid concomitant use of cefazolin and rifampin in patients at increased risk for bleeding. If no alternative treatment options are available, closely monitor prothrombin time and other coagulation tests, and administer vitamin K as indicated.
Isoniazid
(See the boxed WARNING.)
Since RIFAMATE contains isoniazid, ophthalmologic examinations (including ophthalmoscopy) should be done before treatment is started and periodically thereafter, even without occurrence of visual symptoms.
Severe cutaneous reactions including Stevens-Johnson syndrome (SJS) and Toxic Epidermal Necrolysis (TEN), some with a fatal outcome, have been reported with the use of isoniazid (see ADVERSE REACTIONS). Monitor for skin reactions and advise patients to report skin rashes or mucosal lesions immediately. Discontinue RIFAMATE if these reactions occur.
-
PRECAUTIONS
General
RIFAMATE should be used with caution in patients with a history of diabetes mellitus, as diabetes management may be more difficult.
Rifampin
For the treatment of tuberculosis, rifampin is usually administered on a daily basis. Doses of rifampin greater than 600 mg given once or twice weekly have resulted in a higher incidence of adverse reactions, including the "flu syndrome" (fever, chills, and malaise), hematopoietic reactions (leukopenia, thrombocytopenia, or acute hemolytic anemia), cutaneous, gastrointestinal, and hepatic reactions, shortness of breath, shock, anaphylaxis, and renal failure. Recent studies indicate that regimens using twice-weekly doses of rifampin 600 mg plus isoniazid 15 mg/kg are much better tolerated.
Rifampin is not recommended for intermittent therapy; the patient should be cautioned against intentional or accidental interruption of the daily dosage regimen since rare renal hypersensitivity reactions have been reported when therapy was resumed in such cases.
Rifampin has enzyme induction properties that can enhance the metabolism of endogenous substrates including adrenal hormones, thyroid hormones, and vitamin D.
Isoniazid
All drugs should be stopped and an evaluation of the patient should be made at the first sign of a hypersensitivity reaction.
Use of RIFAMATE, because it contains isoniazid, should be carefully monitored in the following:
- Patients who are receiving phenytoin (diphenylhydantoin) concurrently. Isoniazid may decrease the excretion of phenytoin or may enhance its effects. To avoid phenytoin intoxication, appropriate adjustment of the anticonvulsant dose should be made.
- Daily users of alcohol. Daily ingestion of alcohol may be associated with a higher incidence of isoniazid hepatitis.
- Patients with current chronic liver disease or severe renal dysfunction.
Information for Patients
Food Interactions
Because isoniazid has some monoamine oxidase inhibiting activity, an interaction with tyramine-containing foods (cheese, red wine) may occur. Diamine oxidase may also be inhibited, causing exaggerated response (e.g., headache, sweating, palpitations, flushing, hypotension) to foods containing histamine (e.g., skipjack, tuna, other tropical fish). Tyramine and histamine-containing foods should be avoided in patients receiving RIFAMATE.
RIFAMATE, because it contains rifampin, may produce a discoloration (yellow, orange, red, brown) of the teeth, urine, sweat, sputum, and tears, and the patient should be forewarned of this. Soft contact lenses may be permanently stained.
Rifampin is a well characterized and potent inducer of drug metabolizing enzymes and transporters and might therefore decrease concomitant drug exposure and efficacy (see DRUG INTERACTIONS). Therefore, patients should be advised not to take any other medication without medical advice.
Patients should be advised that the reliability of oral or other systemic hormonal contraceptives may be affected; consideration should be given to using alternative contraceptive measures.
Patients should be instructed to take RIFAMATE either 1 hour before or 2 hours after a meal with a full glass of water.
Patients should be instructed to notify their physician immediately if they experience any of the following: rash with fever or blisters, with or without peeling skin, itching, or swollen lymph nodes, loss of appetite, malaise, nausea, vomiting, abdominal pain, darkened urine, yellowish discoloration of the skin and eyes, light-colored bowel movements, cough, shortness of breath, wheezing, and pain or swelling of the joints.
Advise patients to abstain from alcohol, hepatotoxic medications or herbal products while taking RIFAMATE.
Compliance with the full course of therapy must be emphasized, and the importance of not missing any doses must be stressed.
Laboratory Tests
Adults treated for tuberculosis with RIFAMATE should have baseline measurements of hepatic enzymes, bilirubin, serum creatinine, a complete blood count (CBC) and platelet count (or estimate), and blood uric acid.
Patients should be seen at least monthly during therapy and should be specifically questioned concerning symptoms associated with adverse reactions. All patients with abnormalities should have follow-up, including laboratory testing, if necessary. Routine laboratory monitoring for toxicity in people with normal baseline measurements is generally not necessary.
Drug Interactions
Rifampin
Pharmacodynamic Interactions
Healthy subjects who received rifampin 600 mg once daily concomitantly with saquinavir 1000 mg/ritonavir 100 mg twice daily (ritonavir-boosted saquinavir) developed severe hepatocellular toxicity. Concomitant use of these medications is contraindicated. (See CONTRAINDICATIONS.)
When rifampin is given concomitantly with other hepatotoxic medications such as halothane or isoniazid, the potential for hepatotoxicity is increased. Avoid concomitant use of RIFAMATE with halothane. Monitor patients receiving RIFAMATE for hepatotoxicity. (See the boxed WARNING.)
Effect of Rifampin on Other Drugs
Induction of drug metabolizing enzymes and transporter systems
Drug metabolizing enzymes and transporters affected by rifampin include cytochromes P450 (CYP) 1A2, 2B6, 2C8, 2C9, 2C19, and 3A4, UDP-glucuronyltransferases (UGT), sulfotransferases, carboxylesterases, and transporters including P-glycoprotein (P-gp) and multidrug resistance-associated protein 2 (MRP2). Most drugs are substrates for one or more of these enzyme or transporter pathways and these pathways may be induced by rifampin simultaneously. Therefore, rifampin may accelerate the metabolism and reduce the activity of certain concomitantly used drugs, and has the potential to perpetuate clinically important drug-drug interactions against many drugs and across many drug classes (Table 1).
Table 1 summarizes the effect of rifampin on other drugs or drug classes. Adjust dosages of concomitant drugs based on approved drug labeling and if applicable, therapeutic drug monitoring, unless otherwise specified.
Table 1: Drug Interactions with Rifampin that Affect Concomitant Drug Concentrations* Drug or Drug Class and Prevention or Management Clinical Effect AUC = area under the time-concentration curve - * Administered with rifampin 600 mg daily, unless otherwise specified
- † Rifampin dosage used concomitantly with the drug(s) is not specified in the proposed package insert.
- ‡ Administered with rifampin 300 mg daily
- § Administered with rifampin 450 mg daily
- ¶ Administered with rifampin 1200 mg daily
- # Rifampin 1200 mg administered as a single oral dose 8 hours before administering a single oral dose of nifedipine 10 mg
- Þ Numerous cases in the literature describe a decrease in glucocorticoid effect when used concomitantly with rifampin. The literature contains reports of acute adrenal crisis or adrenal insufficiency induced by the combination of rifampin-isoniazid-ethambutol or rifampin-isoniazid in patients with Addison's disease.
- ß Administered with rifampin 900 mg daily
- à A tuberculosis treatment regimen including rifampin (600 mg/day), isoniazid (300 mg/day), pyrazinamide (500 mg 3× per day), and pyridoxine (25 mg) was associated with higher than expected doses of nortriptyline were required to obtain a therapeutic drug level. Following the discontinuation of rifampin, the patient became drowsy and the serum nortriptyline levels rose precipitously (3-fold) into the toxic range.
- è Concomitant use with rifampin in 2 children
- ð Administered with rifampin (10 mg/kg daily)
- ø Administered with an antibiotic regimen including rifampin (450 mg/day), isoniazid (300 mg/day), and streptomycin (0.5 g/day) IM
Antiretrovirals
Prevention or Management: Concomitant use is contraindicated (See CONTRAINDICATIONS)Atazanavir Decrease AUC by 72% Darunavir† Substantial decrease in exposure, which may result in loss of therapeutic effect and development of resistance. Tipranavir Fosamprenavir‡ Decrease AUC by 82% Saquinavir Decrease AUC by 70%
Coadministration may result in severe hepatocellular toxicityAntiretrovirals
Prevention or Management: Avoid concomitant useZidovudine Decrease AUC by 47% Indinavir Decrease AUC by 92% Efavirenz Decrease AUC by 26 % Hepatitis C Antiviral
Prevention or Management: Avoid concomitant useDaclatasvir Decrease AUC by 79% Simeprevir Decrease AUC by 48% Sofosbuvir† Decrease AUC by 72%
Coadministration of sofosbuvir with rifampin, may decrease sofosbuvir plasma concentrations, leading to reduced therapeutic effect of sofosbuvir.Telaprevir Decrease AUC by 92% Systemic Hormonal Contraceptives
Prevention or Management: Advise patients to change to non-hormonal methods of birth control during rifampin therapyEstrogens Decrease exposure Progestins Anticonvulsants Phenytoin§ Decrease exposure§ Antiarrhythmics Disopyramide Decrease exposure Mexiletine Decrease exposure Quinidine Decrease exposure Propafenone Decrease AUC by 50%–67% Tocainide Decrease exposure Antiestrogens Tamoxifen Decrease AUC by 86% Toremifene Decrease steady state concentrations of toremifene in serum Antipsychotics Haloperidol Decrease plasma concentrations by 70% Oral Anticoagulants
Prevention or Management: Perform prothrombin time daily or as frequently as necessary to establish and maintain the required dose of anticoagulantWarfarin Decrease exposure Antifungals Fluconazole Decrease AUC by 23% Itraconazole
Prevention or Management: Not recommended 2 weeks before and during itraconazole treatmentDecrease exposure Ketoconazole Decrease exposure Beta-blockers Metoprolol Decrease exposure Propranolol Decrease exposure Benzodiazepines Diazepam*,¶ Decrease exposure Benzodiazepine-related drugs Zopiclone Decrease AUC by 82% Zolpidem Decrease AUC by 73% Calcium Channel Blockers¶ Diltiazem Decrease exposure Nifedipine# Decrease exposure Verapamil Decrease exposure CorticosteroidsÞ Prednisolone Decrease exposure Cardiac Glycosides Digoxin
Prevention or Management: Measure serum digoxin concentrations before initiating rifampin. Continue monitoring and increase digoxin dose by approximately 20%–40% as necessary.Decrease exposure Digitoxin Decrease exposure Fluoroquinolones Pefloxacinß Decrease exposure Moxifloxacin*,§ Decrease exposure Oral Hypoglycemic Agents (e.g., sulfonylureas) Glyburide Decrease exposure
Rifampin may worsen glucose control of glyburideGlipizide Decrease exposure Immunosuppressive Agents Cyclosporine Decrease exposure Tacrolimus
Prevention or Management: Monitoring of whole blood concentrations and appropriate dosage adjustments of tacrolimus are recommended when rifampin and tacrolimus are used concomitantly.Decrease AUC by 56% Narcotic Analgesics Oxycodone Decrease AUC by 86% Morphine Decrease exposure Selective 5-HT3 Receptor Antagonists Ondansetron Decrease exposure Statins Metabolized by CYP3A4 Simvastatin Decrease exposure Thiazolidinediones Rosiglitazone Decrease AUC by 66% Tricyclic Antidepressants Nortriptylineà Decrease exposure Other Drugs Enalapril Decrease active metabolite exposure Chloramphenicolè Decrease exposure Clarithromycin Decrease exposure Dapsone Decrease exposure Doxycyclineð Decrease exposure Irinotecanø
Prevention or Management: Avoid the use of rifampin, strong CYP3A4 inducer, if possible. Substitute non-enzyme inducing therapies at least 2 weeks prior to initiation of irinotecan therapyDecrease irinotecan and active metabolite exposure Levothyroxine Decrease exposure Losartan Parent Decrease AUC by 30% Active metabolite (E3174) Decrease AUC by 40%. Methadone In patients well-stabilized on methadone, concomitant administration of rifampin resulted in a marked reduction in serum methadone levels and a concurrent appearance of withdrawal symptoms. Praziquantel
Prevention or Management: Concomitant use is contraindicated (See CONTRAINDICATIONS)Decrease plasma praziquantel concentrations to undetectable levels. Quinine
Prevention or Management: Avoid concomitant useDecrease AUC by 75%–85% Telithromycin Decrease AUC by 86% Theophylline Decrease exposure by 20% to 40% Effect of Other Drugs on Rifampin
Concomitant use with antacids may reduce the absorption of rifampin which may reduce the efficacy of RIFAMATE. Administer RIFAMATE at least 1 hour before the ingestion of antacids.
Concomitant use with probenecid and cotrimoxazole increase the concentration of rifampin which may increase the risk of RIFAMATE toxicities. Monitor for adverse reactions associated with in RIFAMATE during coadministration.
Isoniazid
Pharmacodynamic Interactions
Concomitant use with daily ingestion of alcohol may be associated with a higher incidence of isoniazid hepatitis. Concomitant use of isoniazid with rifampin may increase the hepatotoxicity of both drugs. Monitor patients receiving both rifampin and isoniazid as in RIFAMATE for hepatotoxicity.
Concomitant use may exaggerate the CNS effects of meperidine (drowsiness), cycloserine (dizziness, drowsiness), and disulfiram (acute behavioral and coordination changes).
Concomitant use with levodopa may produce symptoms of excess catecholamine stimulation (agitation, flushing, palpitations) or lack of levodopa effect.
Concomitant use with oral hypoglycemics may produce hyperglycemia and lead to loss of glucose control.
Concomitant use with enflurane may produce high concentrations of hydrazine that facilitate defluorination of enflurane due to fast acetylation of isoniazid. Monitor renal function.
Pharmacokinetic Interactions
Effect of Isoniazid on Other Drugs
Inhibition of drug metabolizing enzymes
Isoniazid is known to inhibit certain cytochrome P-450 enzymes (e.g., CYP1A2, CYP2C9, CYP2C19, CYP3A4). Concomitant use may decrease elimination of drugs metabolized by these enzymes which may increase the risk of toxicities of these drugs. Adjust dosages of drugs metabolized by these enzymes based on approved drug labeling and if applicable, therapeutic drug monitoring.
Isoniazid has been reported to inhibit the metabolism of the following drugs: anticonvulsants (e.g., carbamazepine, phenytoin, primidone, valproic acid), benzodiazepines (e.g., diazepam), haloperidol, ketoconazole, theophylline, and warfarin. Therefore, isoniazid may increase the risk of toxicities of these drugs. Adjust dosages of drugs metabolized by these enzymes based on approved drug labeling and if applicable, therapeutic drug monitoring. Concomitant use with RIFAMATE, which also contains rifampin (inducer), on the metabolism of these drugs is unknown.
Other Interactions
Antacid
Concomitant use with antacid may reduce the absorption of isoniazid which may reduce RIFAMATE efficacy. Administer RIFAMATE at least 1 hour before use of antacids.
Drug/Laboratory Test Interactions
Rifampin
Cross-reactivity and false-positive urine screening tests for opiates have been reported in patients receiving rifampin when using the KIMS (Kinetic Interaction of Microparticles in Solution) method (e.g., Abuscreen OnLine opiates assay; Roche Diagnostic Systems). Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish rifampin from opiates.
Therapeutic levels of rifampin have been shown to inhibit standard microbiological assays for serum folate and vitamin B12. Therefore, alternative assay methods should be considered. Transient abnormalities in liver function tests (e.g., elevation in serum bilirubin, alkaline phosphatase and serum transaminases), and reduced biliary excretion of contrast media used for visualization of the gallbladder have also been observed. Therefore, these tests should be performed before the morning dose of RIFAMATE.
Rifampin and isoniazid have been reported to alter vitamin D metabolism. In some cases, reduced levels of circulating 25-hydroxy vitamin D and 1,25-dihydroxy vitamin D have been accompanied by reduced serum calcium and phosphate, and elevated parathyroid hormone.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Increased frequency of chromosomal aberrations was observed in vitro in lymphocytes obtained from patients treated with combinations of rifampin, isoniazid, and pyrazinamide and combinations of streptomycin, rifampin, isoniazid, and pyrazinamide.
Rifampin
A few cases of accelerated growth of lung carcinoma have been reported in man, but a causal relationship with the drug has not been established. Hepatomas were increased in female (C3Hf/DP) mice dosed for 60 weeks with rifampin followed by an observation period of 46 weeks, at 20 to 120 mg/kg (equivalent to 0.1 to 0.5 times the maximum dosage used clinically, based on body surface area comparisons). There was no evidence of tumorigenicity in male C3Hf/DP mice or in similar studies in BALB/c mice, or in two year studies in Wistar rats.
There was no evidence of mutagenicity in both prokaryotic (Salmonella typhi, Escherichia coli) and eukaryotic (Saccharomyces cerevisiae) bacteria, Drosophila melanogaster, or ICR/Ha Swiss mice. An increase in chromatid breaks was noted when whole blood cell cultures were treated with rifampin.
Pregnancy
Teratogenic Effects
Although animal reproduction studies have not been conducted with RIFAMATE, teratogenic effects (including cleft palate and spina bifida) have been observed in rodents treated with rifampin at doses 0.2 to 2 times the maximum recommended human dose, based on body surface area comparisons. There are no adequate and well-controlled studies of RIFAMATE in pregnant women. RIFAMATE should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Rifampin
Congenital malformations, primarily spina bifida, were increased in the offspring of pregnant rats given rifampin during organogenesis at oral doses of 150 to 250 mg/kg/day (about 1 to 2 times the maximum recommended human dose based on body surface area comparisons). Cleft palate was increased in a dose-dependent fashion in fetuses of pregnant mice treated at oral doses of 50 to 200 mg/kg (about 0.2 to 0.8 times the maximum recommended human dose based on body surface area comparisons). Imperfect osteogenesis and embryotoxicity were also reported in pregnant rabbits given rifampin at oral doses up to 200 mg/kg/day (about 3 times the maximum recommended daily human dose based on body surface area comparisons). Although there are no adequate and well-controlled studies in pregnant women, rifampin has been reported to cross the placental barrier and appear in cord blood.
Pregnancy
Nursing Mothers
Because of the potential for tumorigenicity shown for rifampin in animal studies, and since rifampin and isoniazid are known to cross the placental barrier and to pass into maternal breast milk, a decision should be made whether to discontinue nursing or to discontinue RIFAMATE, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients under the age of 15 have not been established. (See CLINICAL PHARMACOLOGY, General; See also DOSAGE AND ADMINISTRATION.)
Geriatric Use
Clinical studies of RIFAMATE did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Caution should therefore be observed in using rifampin and isoniazid in elderly patients. (See WARNINGS.)
-
ADVERSE REACTIONS
Rifampin
Gastrointestinal: heartburn, epigastric distress, anorexia, nausea, vomiting, jaundice, flatulence, cramps, and diarrhea have been noted in some patients. Although Clostridium difficile has been shown in vitro to be sensitive to rifampin, pseudomembranous colitis has been reported with the use of rifampin (and other broad spectrum antibiotics). Therefore, it is important to consider this diagnosis in patients who develop diarrhea in association with antibiotic use. Tooth discoloration (which may be permanent) may occur.
Hepatic: hepatotoxicity including transient abnormalities in liver function tests (e.g., elevations in serum bilirubin, alkaline phosphatase, serum transaminases, gamma-glutamyl transferase), hepatitis, a shock-like syndrome with hepatic involvement and abnormal liver function tests, and cholestasis have been reported (see WARNINGS).
Hematologic: thrombocytopenia has occurred primarily with high dose intermittent therapy, but has also been noted after resumption of interrupted treatment. It rarely occurs during well-supervised daily therapy. This effect is reversible if the drug is discontinued as soon as purpura occurs. Cerebral hemorrhage and fatalities have been reported when rifampin administration has been continued or resumed after the appearance of purpura.
Rare reports of disseminated intravascular coagulation have been observed.
Leukopenia, hemolytic anemia, decreased hemoglobin, bleeding, and vitamin K–dependent coagulation disorders (abnormal prolongation of prothrombin time or low vitamin K–dependent coagulation factors) have been observed.
Agranulocytosis has been reported rarely.
Central Nervous System: headache, fever, drowsiness, fatigue, ataxia, dizziness, inability to concentrate, mental confusion, behavioral changes, muscular weakness, pains in extremities, and generalized numbness have been observed.
Psychoses have been rarely reported.
Rare reports of myopathy have also been observed.
Ocular: visual disturbances have been observed.
Endocrine: menstrual disturbances have been observed.
Rare reports of adrenal insufficiency in patients with compromised adrenal function have been observed.
Renal: elevations in BUN and serum uric acid have been reported. Rarely, hemolysis, hemoglobinuria, hematuria, interstitial nephritis, acute tubular necrosis, renal insufficiency, and acute renal failure have been noted. These are generally considered to be hypersensitivity reactions. They usually occur during intermittent therapy or when treatment is resumed following intentional or accidental interruption of a daily dosage regimen, and are reversible when rifampin is discontinued and appropriate therapy instituted.
Dermatologic: cutaneous reactions are mild and self-limiting and do not appear to be hypersensitivity reactions. Typically, they consist of flushing and itching with or without a rash. More serious cutaneous reactions which may be due to hypersensitivity occur but are uncommon.
Hypersensitivity reactions: occasionally pruritus, urticaria, rash, pemphigoid reaction, erythema multiforme, acute generalized exanthematous pustulosis, Stevens-Johnson syndrome, toxic epidermal necrolysis, Drug Reaction with Eosinophilia and Systemic Symptoms syndrome (see WARNINGS), vasculitis, eosinophilia, sore mouth, sore tongue, and conjunctivitis have been observed.
Anaphylaxis has been reported rarely.
Miscellaneous: edema of the face and extremities has been reported. Other reactions which have occurred with intermittent dosage regimens include "flu" syndrome (such as episodes of fever, chills, headache, dizziness, and bone pain), shortness of breath, wheezing, decrease in blood pressure and shock. The "flu" syndrome may also appear if rifampin is taken irregularly by the patient or if daily administration is resumed after a drug-free interval.
Isoniazid
The most frequent reactions are those affecting the nervous system and the liver. (See the boxed WARNING.)
Nervous system: peripheral neuropathy is the most common toxic effect. It is dose-related, occurs most often in the malnourished and in those predisposed to neuritis (e.g., alcoholics and diabetics), and is usually preceded by paresthesia of the feet and hands. The incidence is higher in "slow inactivators."
Other neurotoxic effects, which are uncommon with conventional doses, are convulsions, toxic encephalopathy, optic neuritis and atrophy, memory impairment, and toxic psychosis.
Gastrointestinal: pancreatitis, nausea, vomiting, and epigastric distress.
Hepatic: elevated serum transaminases (SGOT; SGPT), bilirubinemia, bilirubinuria, jaundice, and occasionally severe and sometimes fatal hepatitis. The common prodromal symptoms are anorexia, nausea, vomiting, fatigue, malaise, and weakness. Mild and transient elevation of serum transaminase levels occurs in 10 to 20% of persons taking isoniazid. The abnormality usually occurs in the first 4 to 6 months of treatment but can occur at any time during therapy. In most instances, enzyme levels return to normal with no necessity to discontinue medication. In occasional instances, progressive liver damage occurs, with accompanying symptoms. In these cases, the drug should be discontinued immediately. The frequency of progressive liver damage increases with age. It is rare in persons under 20, but occurs in up to 2.3% of those over 50 years of age.
Hematologic: agranulocytosis, hemolytic sideroblastic or aplastic anemia, thrombocytopenia, and eosinophilia.
Hypersensitivity reactions: fever, skin eruptions (morbilliform, maculopapular, purpuric, or exfoliative), lymphadenopathy, anaphylactic reactions, Stevens-Johnson syndrome, toxic epidermal necrolysis (see WARNINGS, Isoniazid), Drug Reaction with Eosinophilia and Systemic Symptoms syndrome (see WARNINGS), and vasculitis.
Metabolic and endocrine: pyridoxine deficiency, pellagra, hyperglycemia, metabolic acidosis, and gynecomastia.
Miscellaneous: rheumatic syndrome and systemic lupus erythematosus-like syndrome.
-
OVERDOSAGE
Signs and Symptoms
Rifampin
Nausea, vomiting, abdominal pain, pruritus, headache, and increasing lethargy will probably occur within a short time after ingestion; actual unconsciousness may occur with severe hepatic involvement. Transient increases in liver enzymes and/or bilirubin may occur. Brownish-red or orange discoloration of the skin, urine, sweat, saliva, tears, and feces is proportional to amount ingested.
Liver enlargement, possibly with tenderness, can develop within a few hours after severe overdosage, bilirubin levels may increase and jaundice may develop rapidly. Hepatic involvement may be more marked in patients with prior impairment of hepatic function. Other physical findings remain essentially normal. A direct effect upon the hematopoietic system, electrolyte levels, or acid-base balance is unlikely.
Facial or periorbital edema has also been reported in pediatric patients. Hypotension, sinus tachycardia, ventricular arrhythmias, seizures, and cardiac arrest were reported in some fatal cases.
Isoniazid
Isoniazid overdosage produces signs and symptoms within 30 minutes to 3 hours. Nausea, vomiting, dizziness, slurring of speech, blurring of vision, visual hallucinations (including bright colors and strange designs), are among the early manifestations. With marked overdosage, respiratory distress and CNS depression, progressing rapidly from stupor to profound coma, are to be expected, along with severe, intractable seizures. Severe metabolic acidosis, acetonuria, and hyperglycemia are typical laboratory findings.
Acute Toxicity
Rifampin
The minimum acute lethal or toxic dose is not well established. However, nonfatal acute overdoses in adults have been reported with doses ranging from 9 to 12 gm rifampin. Fatal acute overdoses in adults have been reported with doses ranging from 14 to 60 gm. Alcohol or a history of alcohol abuse was involved in some of the fatal and nonfatal reports. Nonfatal overdoses in pediatric patients ages 1 to 4 years old of 100 mg/kg for one to two doses has been reported.
Isoniazid
Untreated or inadequately treated cases of gross isoniazid overdosage can be fatal, but good response has been reported in most patients treated within the first few hours after drug ingestion.
Ingested acutely, as little as 1.5 g isoniazid may cause toxicity in adults. Doses of 35 to 40 mg/kg have resulted in seizures. Ingestion of 80 to 150 mg/kg isoniazid has been associated with severe toxicity and, if untreated, significant mortality.
Treatment
The airway should be secured and adequate respiratory exchange established. Only then should gastric emptying (lavage-aspiration) be attempted; this may be difficult because of seizures. Since nausea and vomiting are likely to be present, gastric lavage is probably preferable to induction of emesis.
Blood samples should be obtained for immediate determination of gases, electrolytes, BUN, glucose, etc. Blood should be typed and cross-matched in preparation for possible hemodialysis.
Gastric lavage within the first 2 to 3 hours after ingestion should not be attempted until convulsions are under control. To treat convulsions, administer IV diazepam or short-acting barbiturates, and IV pyridoxine (usually 1 mg/1 mg isoniazid ingested). Activated charcoal slurry instilled into the stomach following evacuation of gastric contents can help absorb any remaining drug in the GI tract. Antiemetic medication may be required to control severe nausea and vomiting.
RAPID CONTROL OF METABOLIC ACIDOSIS IS FUNDAMENTAL TO MANAGEMENT. Intravenous sodium bicarbonate should be given at once and repeated as needed, adjusting subsequent dosage on the basis of laboratory findings (i.e., serum sodium, pH, etc.).
Forced osmotic diuresis must be started early and should be continued for some hours after clinical improvement to hasten renal clearance of drug and help prevent relapse. Fluid intake and output should be monitored.
Bile drainage may be indicated in presence of serious impairment of hepatic function lasting more than 24–48 hours. Under these circumstances and for severe cases, extracorporeal hemodialysis may be required; if this is not available, peritoneal dialysis can be used along with forced diuresis.
Along with measures based on initial and repeated determination of blood gases and other laboratory tests as needed, meticulous respiratory and other intensive care should be utilized to protect against hypoxia, hypotension, aspiration, pneumonitis, etc.
Untreated or inadequately treated cases of gross isoniazid overdosage can terminate fatally, but good response has been reported in most patients brought under adequate treatment within the first few hours after drug ingestion.
-
DOSAGE AND ADMINISTRATION
A three-drug regimen consisting of rifampin, isoniazid, and pyrazinamide (e.g., RIFATER®) is recommended in the initial phase of short-course therapy which is usually continued for 2 months. The Advisory Council for the Elimination of Tuberculosis, the American Thoracic Society, and Centers for Disease Control and Prevention recommend that either streptomycin or ethambutol be added as a fourth drug in a regimen containing isoniazid (INH), rifampin, and pyrazinamide for initial treatment of tuberculosis unless the likelihood of INH or rifampin resistance is very low. The need for a fourth drug should be reassessed when the results of susceptibility testing are known. If community rates of INH resistance are currently less than 4%, an initial treatment regimen with less than four drugs may be considered.
Following the initial phase, treatment should be continued with RIFAMATE for at least 4 months. Treatment should be continued for longer if the patient is still sputum or culture positive, if resistant organisms are present, or if the patient is HIV positive.
Concomitant administration of pyridoxine (B6) is recommended in the malnourished, in those predisposed to neuropathy (e.g., alcoholics and diabetics), and in adolescents.
See CLINICAL PHARMACOLOGY, General, for dosing information in patients with renal failure.
-
HOW SUPPLIED
Capsules (opaque red), imprinted "RIFAMATE" on both ends of the capsule, containing 300 mg rifampin and 150 mg isoniazid; bottles of 60 (NDC: 0068-0509-60).
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 60 Capsule Bottle Label
-
INGREDIENTS AND APPEARANCE
RIFAMATE
rifampin and isoniazid capsuleProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 0068-0509 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength rifampin (UNII: VJT6J7R4TR) (rifampin - UNII:VJT6J7R4TR) rifampin 300 mg isoniazid (UNII: V83O1VOZ8L) (isoniazid - UNII:V83O1VOZ8L) isoniazid 150 mg Inactive Ingredients Ingredient Name Strength silicon dioxide (UNII: ETJ7Z6XBU4) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) magnesium stearate (UNII: 70097M6I30) sodium starch glycolate type a potato (UNII: 5856J3G2A2) titanium dioxide (UNII: 15FIX9V2JP) Product Characteristics Color RED Score no score Shape CAPSULE Size 14mm Flavor Imprint Code RIFAMATE Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0068-0509-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 07/11/1975 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA061884 07/11/1975 Labeler - sanofi-aventis U.S. LLC (824676584) Establishment Name Address ID/FEI Business Operations Patheon Pharmaceuticals Inc. 005286822 MANUFACTURE(0068-0509) , ANALYSIS(0068-0509) , LABEL(0068-0509) , PACK(0068-0509)
Trademark Results [Rifamate]
Mark Image Registration | Serial | Company Trademark Application Date |
|---|---|
 RIFAMATE 73038149 1025618 Live/Registered |
DOW CHEMICAL COMPANY, THE 1974-11-25 |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
