STOOL SOFTENER- docusate sodium capsule, liquid filled
Stool Softener by
Drug Labeling and Warnings
Stool Softener by is a Otc medication manufactured, distributed, or labeled by L.N.K. International, Inc., Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd, LNK International, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- Active ingredient (in each liquid-filled capsule)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- stomach pain
- nausea
- vomiting
- noticed a sudden change in bowl habits that lasts over 2 weeks
- Directions
- Other information
- Inactive ingredients
- Questions or comments?
-
Principal display panel
Quality
+ PlusNDC: 50844-655-56
*Compare to active ingredient in
Dulcolax® Stool SoftenerSTOOL SOFTENER
Docusate Sodium 100 mg
STOOL SOFTENER LAXATIVEComfortable, Stimulant-Free
Constipation Relief25 Liquid Gels
ACTUAL SIZE
TAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
*This product is not manufactured or distributed by
Boehringer Ingelheim Pharmaceuticals, Inc., owner of
the registered trademark Dulcolax® Stool Softener.50844 ORG011565556
Product of China
Packaged and Quality Assured in the USADistributed by:
LNK INTERNATIONAL, INC.
60 Arkay Drive
Hauppauge, NY 11788
USA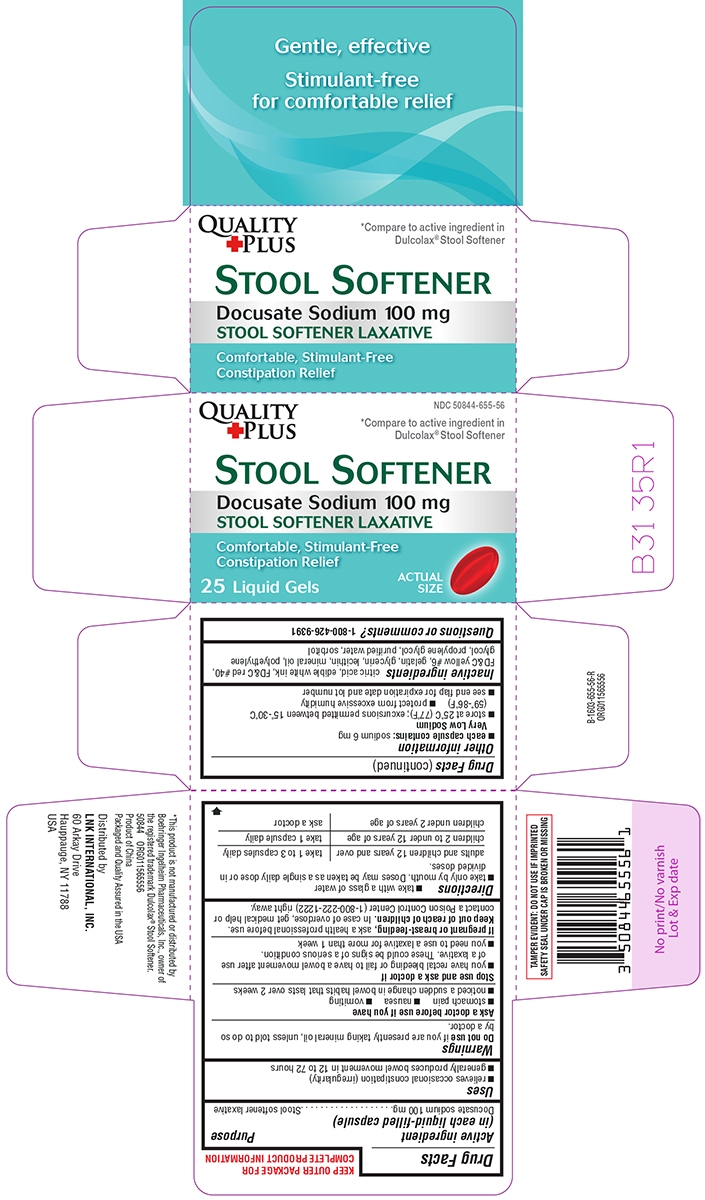
Quality Plus 44-655
-
INGREDIENTS AND APPEARANCE
STOOL SOFTENER
docusate sodium capsule, liquid filledProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC: 50844-655 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DOCUSATE SODIUM (UNII: F05Q2T2JA0) (DOCUSATE - UNII:M7P27195AG) DOCUSATE SODIUM 100 mg Inactive Ingredients Ingredient Name Strength CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) GELATIN (UNII: 2G86QN327L) GLYCERIN (UNII: PDC6A3C0OX) MINERAL OIL (UNII: T5L8T28FGP) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SORBITOL (UNII: 506T60A25R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) WATER (UNII: 059QF0KO0R) Product Characteristics Color ORANGE Score no score Shape OVAL Size 13mm Flavor Imprint Code 655 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 50844-655-56 1 in 1 CARTON 03/01/2015 1 25 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part334 03/01/2015 Labeler - L.N.K. International, Inc. (038154464) Establishment Name Address ID/FEI Business Operations Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd 421293287 API MANUFACTURE(50844-655) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 967626305 PACK(50844-655) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 832867837 PACK(50844-655) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 868734088 PACK(50844-655) Establishment Name Address ID/FEI Business Operations LNK International, Inc. 038154464 PACK(50844-655)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.