RETATRUTIDE PEN injection, solution
RETATRUTIDE Pen by
Drug Labeling and Warnings
RETATRUTIDE Pen by is a Prescription medication manufactured, distributed, or labeled by Guangzhou Yixin Cross-border E-commerce Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
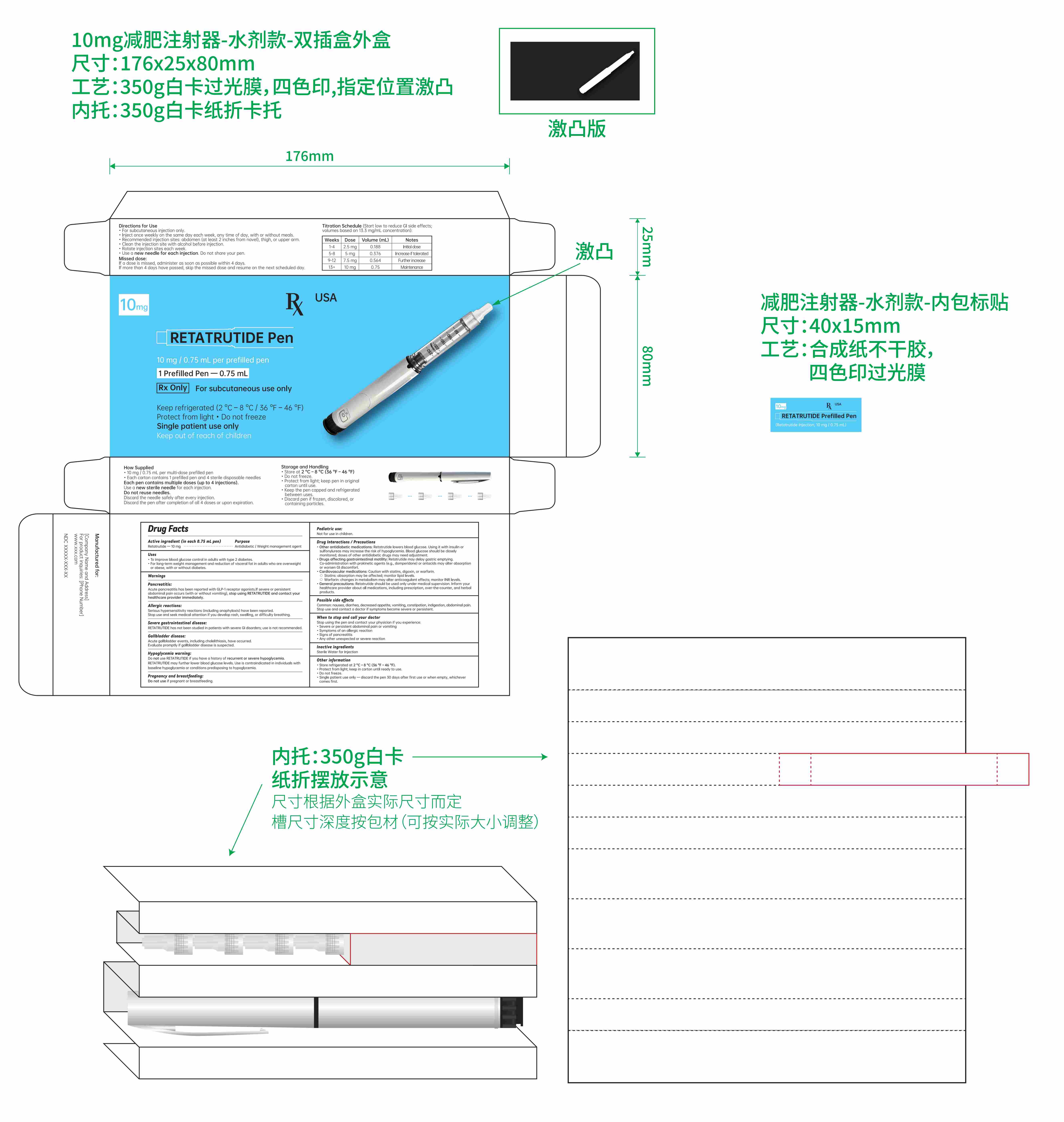
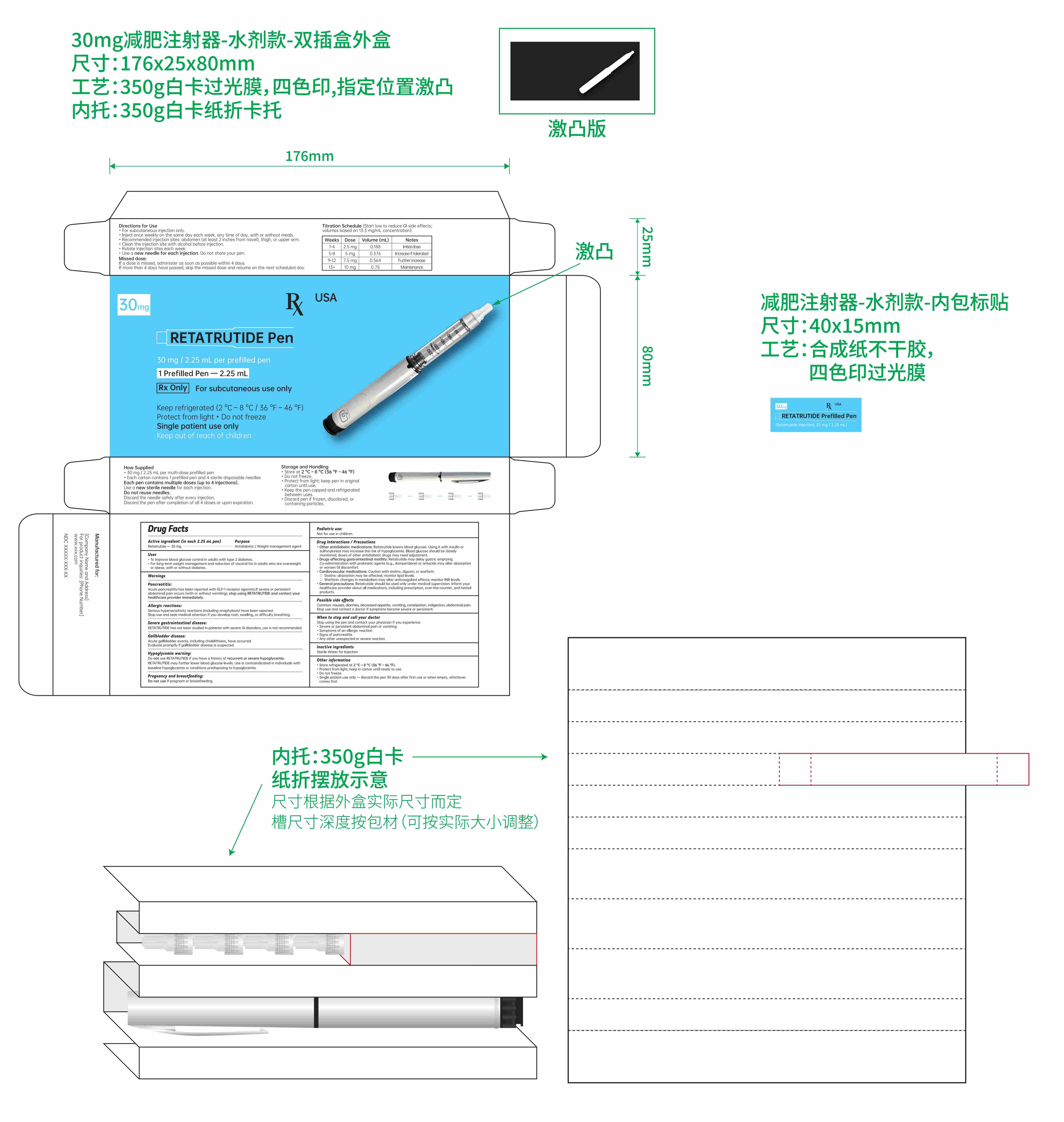
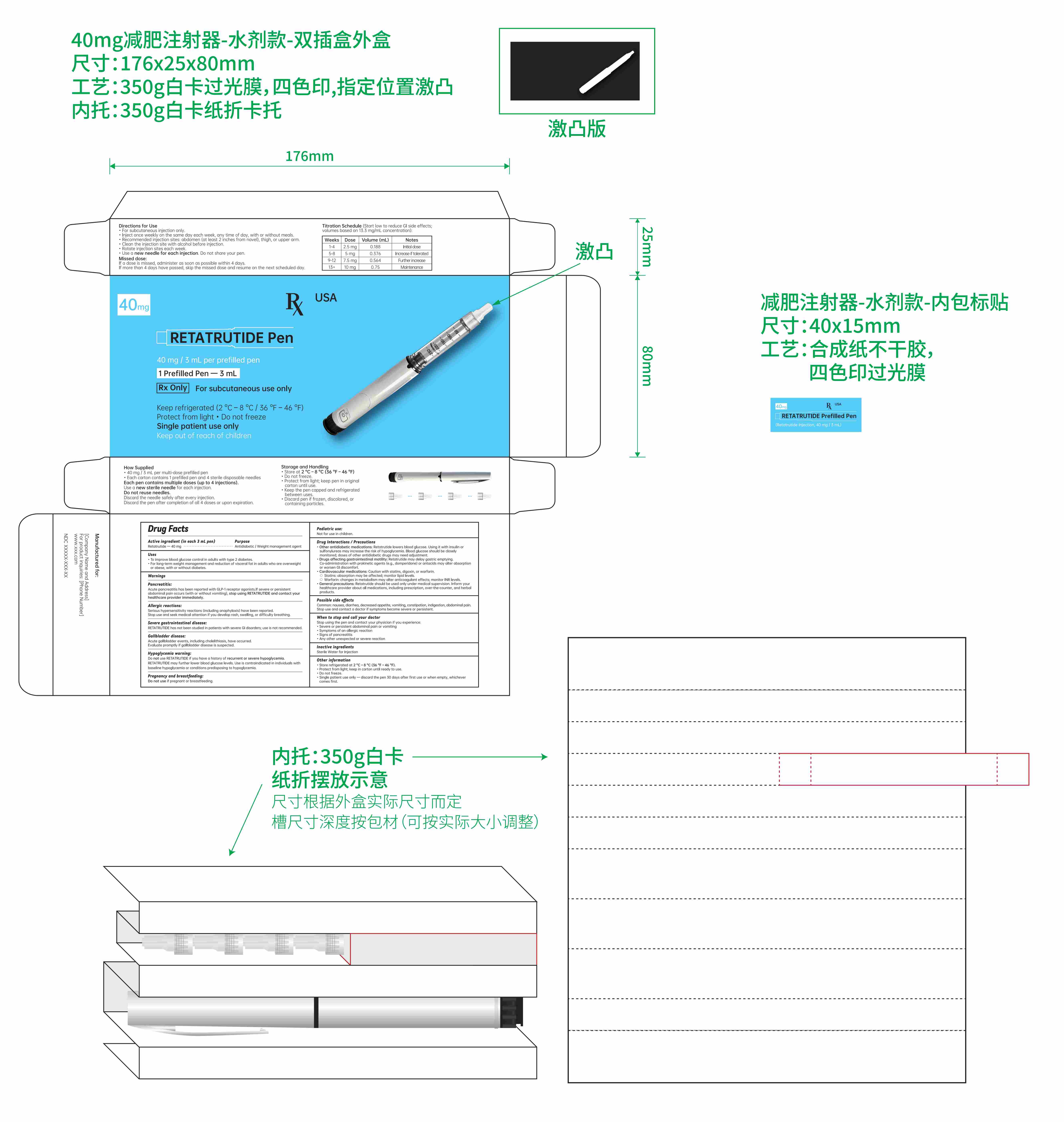
- Active ingredient (in each 0.75 mL pen)
- Purpose
- Uses
-
Warnings
Pancreatitis: Acute pancreatitis has been reported with GLP-1 receptor agonists. If severe or persistent abdominal pain occurs (with or without vomiting), stop using RETATRUTIDE and contact your healthcare provider immediately.
Allergic reactions: Serious hypersensitivity reactions (including anaphylaxis) have been reported.
Stop use and seek medical attention if you develop rash, swelling, or difficulty breathing.
Severe gastrointestinal disease: RETATRUTIDE has not been studied in patients with severe GI disorders; use is not recommended.
Gallbladder disease: Acute gallbladder events, including cholelithiasis, have occurred. Evaluate promptly if gallbladder disease is suspected.
Hypoglycemia warning:
Do not use RETATRUTIDE if you have a history of recurrent or severe hypoglycemia.
RETATRUTIDE may further lower blood glucose levels. Use is contraindicated in individuals with baseline hypoglycemia or conditions predisposing to hypoglycemia.
Pregnancy and breastfeeding: Do not use if pregnant or breastfeeding.
Pediatric use: Not for use in children.
Drug Interactions / Precautions
Other antidiabetic medications: Retatrutide lowers blood glucose. Using it with insulin or sulfonylureas may increase the risk of hypoglycemia. Blood glucose should be closely monitored; doses of other antidiabetic drugs may need adjustment.
Drugs affecting gastrointestinal motility: Retatrutide may delay gastric emptying. Co-administration with prokinetic agents (e.g., domperidone) or antacids may alter absorption or worsen GI discomfort.
Cardiovascular medications: Caution with statins, digoxin, or warfarin.Statins: absorption may be affected; monitor lipid levels.
Warfarin: changes in metabolism may alter anticoagulant effects; monitor INR levels.General precautions: Retatrutide should be used only under medical supervision. Inform your healthcare provider about all medications, including prescription, over-the-counter, and herbal products.
Possible side effects
Common: nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, abdominal pain.
Stop use and contact a doctor if symptoms become severe or persistent.
When to stop and call your doctor
Stop using the pen and contact your physician if you experience:
Severe or persistent abdominal pain or vomiting
Symptoms of an allergic reaction
Signs of pancreatitis
Any other unexpected or severe reaction
- Titration schedule:
-
Directions for Use
For subcutaneous injection only.
Inject once weekly on the same day each week, any time of day, with or without meals.
Recommended injection sites: abdomen, thigh, or upper arm.
Clean the injection site with alcohol before injection.
Rotate injection sites each week.
Use a new needle for each injection. Do not share your pen.
-
Uses
To improve blood glucose control in adults with type 2 diabetes.
For long-term weight management and reduction of visceral fat in adults who are overweight or obese, with or without diabetes.
Warnings
Pancreatitis: Acute pancreatitis has been reported with GLP-1 receptor agonists. If severe or persistent abdominal pain occurs (with or without vomiting), stop using RETATRUTIDE and contact your healthcare provider immediately.
Allergic reactions: Serious hypersensitivity reactions (including anaphylaxis) have been reported.
Stop use and seek medical attention if you develop rash, swelling, or difficulty breathing.
Severe gastrointestinal disease: RETATRUTIDE has not been studied in patients with severe GI disorders; use is not recommended.
Gallbladder disease: Acute gallbladder events, including cholelithiasis, have occurred. Evaluate promptly if gallbladder disease is suspected.
Hypoglycemia warning:
Do not use RETATRUTIDE if you have a history of recurrent or severe hypoglycemia.
RETATRUTIDE may further lower blood glucose levels. Use is contraindicated in individuals with baseline hypoglycemia or conditions predisposing to hypoglycemia.
Pregnancy and breastfeeding: Do not use if pregnant or breastfeeding.
Pediatric use: Not for use in children.
Drug Interactions / Precautions
Other antidiabetic medications: Retatrutide lowers blood glucose. Using it with insulin or sulfonylureas may increase the risk of hypoglycemia. Blood glucose should be closely monitored; doses of other antidiabetic drugs may need adjustment.
Drugs affecting gastrointestinal motility: Retatrutide may delay gastric emptying. Co-administration with prokinetic agents (e.g., domperidone) or antacids may alter absorption or worsen GI discomfort.
Cardiovascular medications: Caution with statins, digoxin, or warfarin.Statins: absorption may be affected; monitor lipid levels.
Warfarin: changes in metabolism may alter anticoagulant effects; monitor INR levels.General precautions: Retatrutide should be used only under medical supervision. Inform your healthcare provider about all medications, including prescription, over-the-counter, and herbal products.
Possible side effects
Common: nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, abdominal pain.
Stop use and contact a doctor if symptoms become severe or persistent.
When to stop and call your doctor
Stop using the pen and contact your physician if you experience:
Severe or persistent abdominal pain or vomiting
Symptoms of an allergic reaction
Signs of pancreatitis
Any other unexpected or severe reaction
-
stop use
Pancreatitis: Acute pancreatitis has been reported with GLP-1 receptor agonists. If severe or persistent abdominal pain occurs (with or without vomiting), stop using RETATRUTIDE and contact your healthcare provider immediately.
Allergic reactions: Serious hypersensitivity reactions (including anaphylaxis) have been reported.
Stop use and seek medical attention if you develop rash, swelling, or difficulty breathing.
Severe gastrointestinal disease: RETATRUTIDE has not been studied in patients with severe GI disorders; use is not recommended.
Gallbladder disease: Acute gallbladder events, including cholelithiasis, have occurred. Evaluate promptly if gallbladder disease is suspected.
Hypoglycemia warning:
Do not use RETATRUTIDE if you have a history of recurrent or severe hypoglycemia.
RETATRUTIDE may further lower blood glucose levels. Use is contraindicated in individuals with baseline hypoglycemia or conditions predisposing to hypoglycemia.
Pregnancy and breastfeeding: Do not use if pregnant or breastfeeding.
Pediatric use: Not for use in children.
Drug Interactions / Precautions
Other antidiabetic medications: Retatrutide lowers blood glucose. Using it with insulin or sulfonylureas may increase the risk of hypoglycemia. Blood glucose should be closely monitored; doses of other antidiabetic drugs may need adjustment.
Drugs affecting gastrointestinal motility: Retatrutide may delay gastric emptying. Co-administration with prokinetic agents (e.g., domperidone) or antacids may alter absorption or worsen GI discomfort.
Cardiovascular medications: Caution with statins, digoxin, or warfarin.Statins: absorption may be affected; monitor lipid levels.
Warfarin: changes in metabolism may alter anticoagulant effects; monitor INR levels.General precautions: Retatrutide should be used only under medical supervision. Inform your healthcare provider about all medications, including prescription, over-the-counter, and herbal products.
Possible side effects
Common: nausea, diarrhea, decreased appetite, vomiting, constipation, indigestion, abdominal pain.
Stop use and contact a doctor if symptoms become severe or persistent.
When to stop and call your doctor
Stop using the pen and contact your physician if you experience:
Severe or persistent abdominal pain or vomiting
Symptoms of an allergic reaction
Signs of pancreatitis
Any other unexpected or severe reaction
- Keep out of reach of children.
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
RETATRUTIDE PEN
retatrutide pen injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 84778-106 Route of Administration SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength RETATRUTIDE (UNII: NOP2Y096GV) (RETATRUTIDE - UNII:NOP2Y096GV) RETATRUTIDE 10 mg in 100 mL Inactive Ingredients Ingredient Name Strength AQUA (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 84778-106-01 4 in 1 BOX 10/29/2025 1 0.75 mL in 1 PAIL; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 2 NDC: 84778-106-02 4 in 1 BOX 10/29/2025 2 2.25 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) 3 NDC: 84778-106-03 4 in 1 BOX 10/29/2025 3 3 mL in 1 SYRINGE; Type 3: Prefilled Biologic Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Export only 10/29/2025 Labeler - Guangzhou Yixin Cross-border E-commerce Co., Ltd. (455800881) Registrant - Guangzhou Yixin Cross-border E-commerce Co., Ltd. (455800881) Establishment Name Address ID/FEI Business Operations Guangzhou Yixin Cross-border E-commerce Co., Ltd. 455800881 label(84778-106) , manufacture(84778-106)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.