VTING by VTING / Natural Korea Co., Ltd. 81222-001_VTING
VTING by
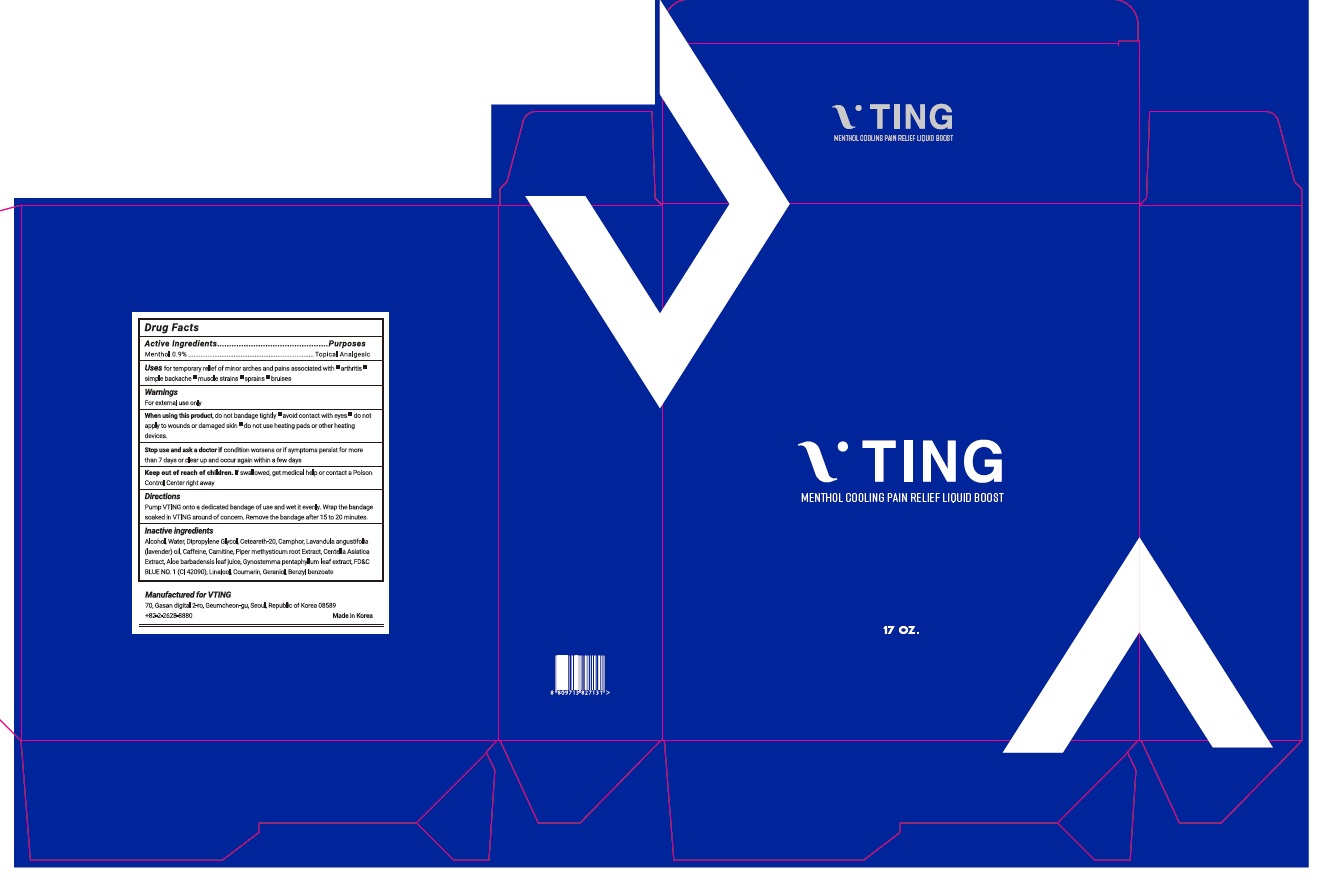
Drug Labeling and Warnings
VTING by is a Otc medication manufactured, distributed, or labeled by VTING, Natural Korea Co., Ltd.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
VTING- menthol liquid
VTING
Disclaimer: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
----------
81222-001_VTING
Uses
Temporary relieves minor aches and pains of muscle and joints associated with backache, arthritis, strains, bruises and sprains.
Warnings
For external use only
Directions
Adults and children 2 years of age and older: Apply to affected areas not more than 3 to 4 times daily.
Children under 2 years of age: consult a physician.
Inactive ingredients
Alcohol, Water, Dipropylene Glycol, Ceteareth-20, Camphor, Lavandula angustifolia (lavender) oil, Caffeine, Carnitine, Piper methysticum root Extract, Centella Asiatica Extract, Aloe barbadensis leaf juice, Gynostemma pentaphyllum leaf extract, FD&C BLUE NO. 1 (CI 42090), Linalool, Coumarin, Geraniol, Benzyl benzoate
| VTING
menthol liquid |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - VTING (695126440) |
| Registrant - VTING (695126440) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Natural Korea Co., Ltd. | 688729438 | manufacture(81222-001) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.