Guaifenesin and Dextromethorphan hydrobromide
Guaifenesin and Dextromethorphan hydrobromide by
Drug Labeling and Warnings
Guaifenesin and Dextromethorphan hydrobromide by is a Otc medication manufactured, distributed, or labeled by Oncor Pharmaceuticals, Quality CDMO. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
GUAIFENESIN AND DEXTROMETHORPHAN HYDROBROMIDE- guaifenesin and dextromethorphan hydrobromide liquid
Oncor Pharmaceuticals
----------
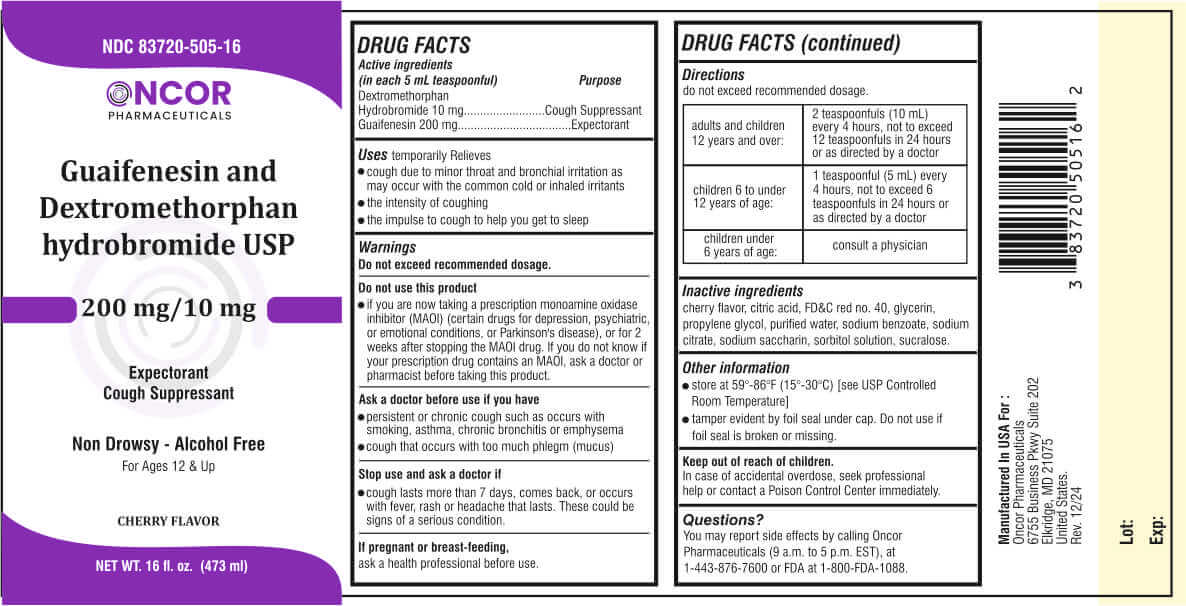
Guaifenesin and Dextromethorphan hydrobromide
Active ingredients
(in each 5 mL teaspoonful) Purpose
Dextromethorphan
Hydrobromide 10 mg..........Cough Suppressant
Guaifenesin 200 mg.......................Expectorant
Uses
temporarily Relieves
- cough due to minor throat and bronchial irritation as may occur with the common cold or inhaled irritants
- the intensity of coughing
- the impulse to cough to help you get to sleep
Do not use this product
If you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson's disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Ask a doctor before use if you have
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis or emphysema
- cough that occurs with too much phlegm (mucus)
Stop use and ask a doctor if
- cough lasts more than 7 days, comes back, or occurs with fever, rash or headache that lasts. These could be signs of a serious condition.
Directions
do not exceed recommended dosage.
| adults and children 12 years and over: | 2 teaspoonfuls (10 mL) every 4 hours, not to exceed 12 teaspoonfuls in 24 hours or as directed by a doctor |
| children 6 to under 12 years of age: | 1 teaspoonful (5 mL) every 4 hours, not to exceed 6 teaspoonfuls in 24 hours or as directed by a doctor |
| children under 6 years of age: | consult a physician |
Inactive ingredients
cherry flavor, citric acid, FD&C red no. 40, glycerin, propylene glycol, purified water, sodium benzoate, sodium citrate, sodium saccharin, sorbitol solution, sucralose.
Other information
- store at 59°-86°F (15°-30°C) [see USP Controlled Room Temperature]
- tamper evident by foil seal under cap. Do not use if foil seal is broken or missing.
In case of accidental overdose, seek professional help or contact a Poison Control Center immediately.
You may report side effects by calling Oncor Pharmaceuticals (9 a.m. to 5 p.m. EST), at 1-443-876-7600 or FDA at 1-800-FDA-1088.
Principal Display Panel
Oncor Pharmaceuticals
NDC: 83720-505-16
Guaifenesin and Dextromethorphan hydrobromide USP
200 mg/10 mg
Expectorant
Cough Suppressant
Non Drowsy - Alcohol Free
For Ages 12 & Up
CHERRY FLAVOR
NET WT. 16 fl. oz. (473 ml)
NDC: 83720-505-04
Guaifenesin and Dextromethorphan hydrobromide USP
200 mg/10 mg
Expectorant
Cough Suppressant
Non Drowsy - Alcohol Free
For Ages 12 & Up
CHERRY FLAVOR
NET WT. 4 OZ. (118 ml)
Manufactured In USA For :
Oncor Pharmaceuticals
6755 Business Pkwy Suite 202
Elkridge, MD 21075
United States.
Rev. 12/24
| GUAIFENESIN AND DEXTROMETHORPHAN HYDROBROMIDE
guaifenesin and dextromethorphan hydrobromide liquid |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Oncor Pharmaceuticals (119032580) |
| Registrant - Oncor Pharmaceuticals (119032580) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Quality CDMO | 117658386 | manufacture(83720-505) | |
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.