MINCORA- vitamin tablet, coated
Mincora by
Drug Labeling and Warnings
Mincora by is a Other medication manufactured, distributed, or labeled by Allegis Pharmaceuticals, LLC. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
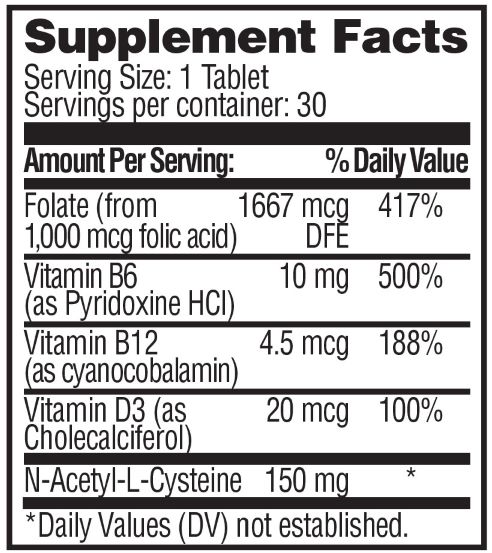
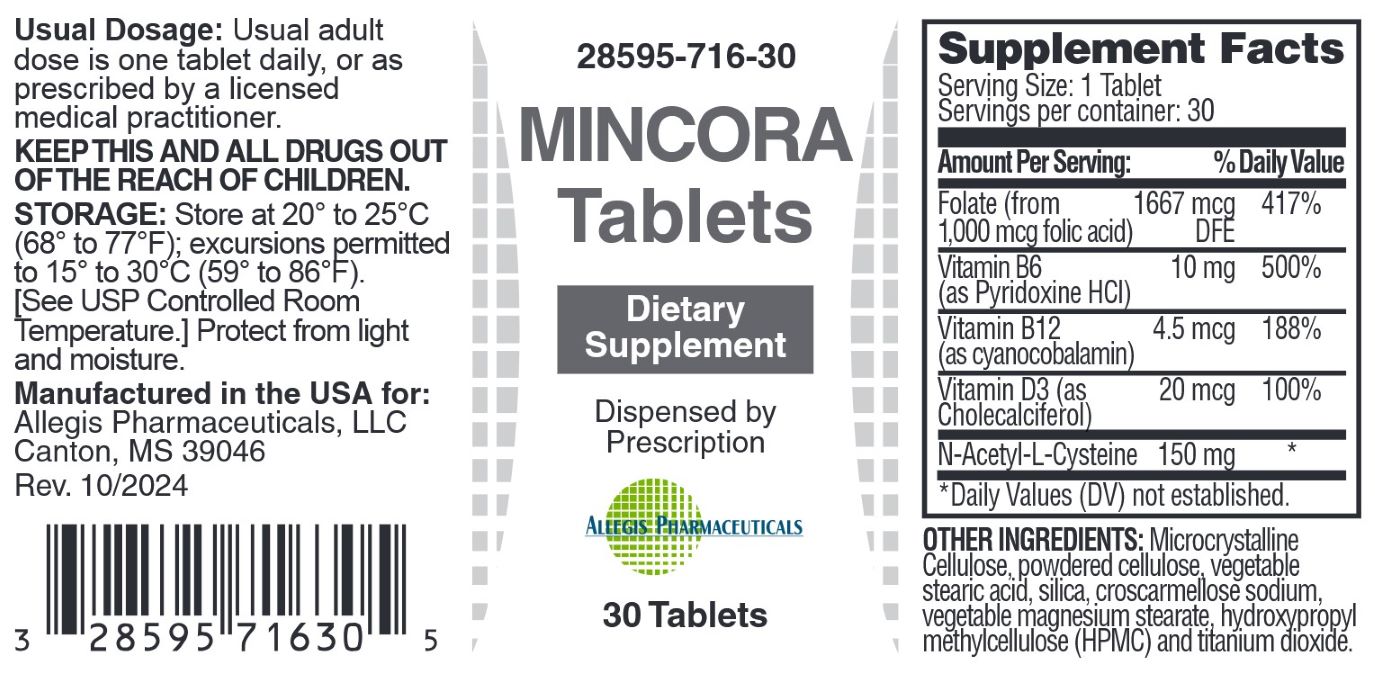
- Suplement Facts
-
Precautions
Folic acid alone is improper therapy in the treatment of pernicious anemia and other megaloblastic anemias where vitamine B12 is deficient.
Folic acid in doses above 0.1 mg daily may obscure pernicious anemia in that hematologic remission can occur while neurological manifestations progress. Mincora Tablets should only be used under the direction and supervision of a licensed medical practitioner.
-
Warnings
KEEP THIS AND ALL DRUGS OUT OF THE REACH OF CHILDREN.
In case of accidental overdose, call a licensed medical practitioner or poison control center immediately.
For use under the supervision of a licensed medical practitioner.
Dispense in a tight, light resistant container as defined in the USP/NF in a child-resistant closure.
-
Dosage and Adminstration
Description: Mincora Tablets is a prescription dietary supplement intended for oral adminstration.
Indication and Usage: Mincora Tablets is to privide significant amounts of Vitamins B6, B12, D3, and folate to supplement the diet, and to help assure that nutritional deficiencies of these vitaines will not develop.
Dosage: Usual adult dose is one tablet daily, or as prescribed by a licensed medical practitioner.
-
Storage
Store at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature.] Protect from light and moisture.Tamper Evident: Do not use if seal if broken or missing.
Statements
† This product is a prescription-folate with or without other dietary ingredients that – due to increased folate levels increased risk associated with masking of B12 deficiency (pernicious anemia) requires administration under the care of a licensed medical practitioner (61 FR 8760). 1-3 The most appropriate way to ensure pedigree reporting consistent with these regulatory guidelines and safety monitoring is to dispense this product only by prescription. This is not an Orange Book product. This product may be administered only under a physician’s supervision and all prescriptions using this product shall be pursuant to state statutes as applicable.
The ingredients, indication or claims of this product are not to be construed to be drug claims.
1. Federal Register Notice of August 2, 1973 (38 FR20750) 2. Federal Register Notice of October 17, 1980 (45 FR69043, 69044) 3. Federal Register Notice of March 5, 1996 (61 FR 8760)
- Label
-
How Supplied
Mincora Tablets: Each bottle contains 30 tablets (white) (28595-716-30)
Mincora Tablets are avaiable as white, round tablets with F100 imprint and are available in 30 count bottles (28595-716-30)
These Statements have note been evaluated by the FDA. This product is not intended to diagnose, treat, cure, or prevent any disease.
Manufactured in the USA for:
Allegis Pharmaceuticals, LLC
Canton, MS 39046
-
INGREDIENTS AND APPEARANCE
MINCORA
vitamin tablet, coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:28595-716 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 4.5 ug CHOLECALCIFEROL (UNII: 1C6V77QF41) (CHOLECALCIFEROL - UNII:1C6V77QF41) CHOLECALCIFEROL 20 ug PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1667 ug N-ACETYL-L-CYSTEINE 2-DEOXY-3-THIO-D-ERYTHRO-PENTONIC ACID .GAMMA.-LACTONE THIOETHER (UNII: KX2B81V402) (N-ACETYL-L-CYSTEINE 2-DEOXY-3-THIO-D-ERYTHRO-PENTONIC ACID .GAMMA.-LACTONE THIOETHER - UNII:KX2B81V402) N-ACETYL-L-CYSTEINE 2-DEOXY-3-THIO-D-ERYTHRO-PENTONIC ACID .GAMMA.-LACTONE THIOETHER 150 mg Inactive Ingredients Ingredient Name Strength CELLULOSE ACETATE (UNII: 3J2P07GVB6) STEARIC ACID (UNII: 4ELV7Z65AP) SILICA DIMETHYL SILYLATE (UNII: EU2PSP0G0W) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) HYDROXYPROPYL METHYLCELLULOSE (UNII: 3NXW29V3WO) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:28595-716-30 30 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 01/06/2025 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape size (solid drugs) 11 mm imprint scoring 1 Labeler - Allegis Pharmaceuticals, LLC (792272861)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.