METAPROTERENOL SULFATE syrup
Metaproterenol Sulfate by
Drug Labeling and Warnings
Metaproterenol Sulfate by is a Prescription medication manufactured, distributed, or labeled by Lannett Company, Inc.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
DESCRIPTION
Metaproterenol sulfate syrup is an oral bronchodilator.
Each teaspoonful (5 mL), for oral administration, contains metaproterenol sulfate 10 mg. In addition, each teaspoonful (5 mL) contains the following inactive ingredients:
citric acid, edetate disodium, FD&C Red No. 40, glycerin, hydroxyethyl cellulose, black cherry flavor, propylene glycol, saccharin sodium, sodium benzoate, sorbitol solution, sodium citrate and purified water.
Metaproterenol sulfate, 1- (3, 5 dihydroxyphenyl) -2-isopropyl - aminoethanol sulfate, is a white, crystalline, racemic mixture of two optically active isomers. It has the following structural formula:
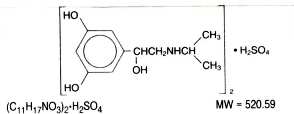
-
CLINICAL PHARMACOLOGY
In vitro studies and in vivo pharmacologic studies have demonstrated that metaproterenol sulfate has a preferential effect on beta2 adrenergic receptors compared with isoproterenol. While it is recognized that beta2 adrenergic receptors are the predominant receptors in bronchial smooth muscle, recent data indicate that there is a population of beta2 receptors in the human heart existing in a concentration between 10 to 50%. The precise function of these, however, is not yet established (see WARNINGS section).
The pharmacologic effects of beta adrenergic agonist drugs, including metaproterenol, are at least in part attributable to stimulation through beta adrenergic receptors of intracellular adenyl cyclase, the enzyme which catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3’, 5’ -adenosine monophosphate (c-AMP). Increased c-AMP levels are associated with relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
PHARMACOKINETICS: Absorption, biotransformation and excretion studies in humans following oral administration have indicated that an average of less than 10% of the drug is absorbed intact; it is not metabolized by catechol-0-methyltransferase nor converted to glucuronide conjugates but is excreted primarily as the sulfate conjugate formed in the gut.
Pulmonary function tests performed after the administration of metaproterenol usually show improvement, e.g., an increase in the one-second forced expiratory volume (FEV1), maximum expiratory flow rate, peak expiratory flow rate, forced vital capacity, and/or a decrease in airway resistance. The resultant decrease in airway obstruction may relieve the dyspnea associated with bronchospasm.
Pulmonary function has been monitored in controlled single- and multiple-dose studies. The duration of effect of a single dose of metaproterenol sulfate syrup (that is, the period of time during which there is a 15% or greater increase in mean FEV1) was up to 4 hours. Recent studies in laboratory animals (minipigs, rodents and dogs) recorded the occurrence of cardiac arrhythmias and sudden death (with histologic evidence of myocardial necrosis) when beta agonists and methylxanthines were administered concurrently. The significance of these findings when applied to humans is currently unknown.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
- WARNINGS
-
PRECAUTIONS
General: Extreme care must be exercised with respect to the administration of additional sympathomimetic agents.
Since metaproterenol is a sympathomimetic amine, it should be used with caution in patients with cardiovascular disorders, including ischemic heart disease, hypertension or cardiac arrhythmias, in patients with hyperthyroidism or diabetes mellitus, and in patients who are unusually responsive to sympathomimetic amines or who have convulsive disorders. Significant changes in systolic and diastolic blood pressure could be expected to occur in some patients after use of any beta adrenergic bronchodilator.
Information For Patients: Appropriate care should be exercised when considering the administration of additional sympathomimetic agents. A sufficient interval of time should elapse prior to administration of another sympathomimetic agent. Metaproterenol should not be used more often than prescribed. If symptoms persist, patients should consult a physician promptly.
Drug Interactions: Other beta adrenergic bronchodilators should not be used concomitantly with metaproterenol because they may have additive effects. Beta adrenergic agonist should be administered with caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants, since the action of beta adrenergic agonists on the vascular system may be potentiated.
Pregnancy: Teratogenic Effects: PREGNANCY CATEGORY C: Metaproterenol sulfate has been shown to be teratogenic and embryotoxic in rabbits when given in doses corresponding to 62 times the maximum recommended dose. These effects included skeletal abnormalities, hydrocephalus and skull bone separation. Results of other studies in rabbits, rats or mice have not revealed any teratogenic, embryotoxic or fetotoxic effects. There are no adequate and well-controlled studies in pregnant women. Metaproterenol sulfate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
-
ADVERSE REACTIONS
Adverse reactions are similar to those noted with other sympathomimetic agents.
The following table of adverse experiences is derived from 44 clinical trials involving 1,120 patients treated with metaproterenol sulfate syrup:
Metaproterenol Sulfate Syrup Incidence of Adverse Events Occurring in at least 1% of Patients Adverse Experience
Incidence
Number of Patients
% Cardiovascular
Tachycardia
68
6.1
Central Nervous System
Headache
12
1.1
Nervousness
54
4.8
Gastrointestinal
Nausea
15
1.3
Musculoskeletal
Tremor
18
1.6
-
OVERDOSAGE
The expected symptoms with overdosage are those of excessive beta stimulation and/or any of the symptoms listed under ADVERSE REACTIONS, e.g., angina, hypertension or hypotension. arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, nausea, dizziness, fatigue, malaise and insomnia.
The treatment consists of discontinuation of metaproterenol together with appropriate symptomatic therapy.
-
DOSAGE AND ADMINISTRATION
Metaproterenol sulfate syrup contains 10 mg of metaproterenol sulfate per teaspoonful (5 mL).
Children: Aged six to nine years or weight under 60 lb - one teaspoonful (5 mL) three or four times a day. Children over nine years or weight over 60 lb - two teaspoonfuls (10 mL) three or four times a day. Clinical trial experience in children under the age of six is limited. Of 40 children treated with metaproterenol sulfate syrup for at least one month, daily doses of approximately 1.3 to 2.6 mg/kg were well tolerated.
Adults: Two teaspoonfuls (10 mL) three or four times a day.
It is recommended that the physician titrate the dosage according to each individual patient’s response to therapy.
-
HOW SUPPLIED
Metaproterenol Sulfate Syrup, USP is available as a red, cherry-flavored syrup containing 10 mg of metaproterenol sulfate per teaspoonful (5 mL) in 473 mL (one pint) bottles.
Store below 86°F (30°C). Protect from light.
Manufactured by:
Silarx Pharmaceuticals, Inc.
1033 Stoneleigh Ave
Carmel, NY 10512 - PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METAPROTERENOL SULFATE
metaproterenol sulfate syrupProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC: 54838-507 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METAPROTERENOL SULFATE (UNII: GJ20H50YF0) (METAPROTERENOL - UNII:53QOG569E0) METAPROTERENOL SULFATE 10 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYETHYL CELLULOSE (2000 CPS AT 1%) (UNII: S38J6RZN16) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SACCHARIN SODIUM (UNII: SB8ZUX40TY) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SODIUM CITRATE (UNII: 1Q73Q2JULR) WATER (UNII: 059QF0KO0R) FD&C RED NO. 40 (UNII: WZB9127XOA) Product Characteristics Color Score Shape Size Flavor CHERRY (Black Cherry) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 54838-507-80 473 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/08/2009 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA073632 09/08/2009 Labeler - Lannett Company, Inc. (161630033)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.
