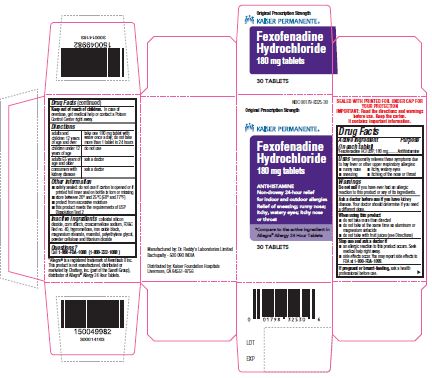
Fexofenadine HCl Tablets USP
Fexofenadine Hydrochloride 180 by
Drug Labeling and Warnings
Fexofenadine Hydrochloride 180 by is a Otc medication manufactured, distributed, or labeled by Kaiser Permanente. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
FEXOFENADINE HYDROCHLORIDE 180- fexofenadine hydrochloride tablet
Kaiser Permanente
----------
Fexofenadine HCl Tablets USP
Use(s)
Allergy
temporarily relieves these symptoms due to hay fever or otherupper respiratory allergies:
- runny nose
- itchy, watery eyes
- sneezing
- itching of the nose or throat
Hives
reduces hives and relieves itching due to hives (urticaria). This product will not prevent hives or an allergic skin reaction from occuring.
Warnings
Hives
Severe Allergic Warning: Get emergency help immediately if you have hives along with any of the following symptom:
- trouble swallowing
- dizziness or loss of consciousness
- swelling of tongue
- swelling in or around mouth
- trouble speaking
- drooling
- wheezing or problems breathing
These symptoms may be signs of anaphylactic shock. This condition canbe life threatening if not treated by a health professional immediately.Symptoms of anaphylactic shock may occur when hives first appear or upto a few hours later.
Not a Substitute for Epinephrine. If your doctor has prescribed an epinephrineinjector for “anaphylaxis” or severe allergy symptoms that could occur withyour hives, never use this product as a substitute for the epinephrine injector.If you have been prescribed an epinephrine injector, you should carry it withyou at all times.
Do not use
Allergy
if you have ever had an allergic reaction to this product or any of its ingredients.
Hives
- to
prevent hives from any known cause such as:
- foods
- insect stings
- medicines
- latex or rubber gloves
because this product will not stop hives from occurring. Avoiding the cause of your hives is the only way to prevent them. Hives can sometimes be serious. If you do not know the cause of your hives, see your doctor for a medical exam. Your doctor may be able to help you find a cause.
- if you have ever had an allergic reaction to this product or any of its ingredients
Ask a doctor before use if you have
Allergy
- kidney disease. Your doctorshould determine if you need a different dose.
Hives
- kidney disease. Your doctor should determine if you need a different dose.
- hives that are an unusual color, look bruised or blistered
- hives that do not itch
When using this product
- do not take more than directed
- do not take at the same time as aluminum or magnesium antacids
- do not take with fruit juices (see Directions)
Directions
180 mg
| adults and children 12 years of age and over | take one 180 mg tablet with water once a day; do not take more than 1 tablet in 24 hours |
| children under 12 years of age | do not use |
| Adults 65 years of age and older | ask a doctor |
| consumers with kidney disease | ask a doctor |
Other information
safety sealed: do not use if carton is opened or if individual blister units are torn or opened.
Storage
store between 20° - 25°C (68° - 77°F)
protect from excessive moisture
this product meets the requirements of USP Dissolution Test 2.
Inactive ingredients
colloidal silicon dioxide, corn starch, croscarmellose sodium, magnesium stearate, mannitol, and powdered cellulose, opadry pink 03B54504 containing FD&C Red no. 40, hypromellose, iron oxide black, polyethylene glycol and titanium dioxide.
| FEXOFENADINE HYDROCHLORIDE 180
fexofenadine hydrochloride tablet |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Kaiser Permanente (053052619) |