FERROUS SULFATE tablet, film coated
Ferrous Sulfate by
Drug Labeling and Warnings
Ferrous Sulfate by is a Other medication manufactured, distributed, or labeled by Rising Pharma Holdings, Inc., Nucrogene Inc. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
-
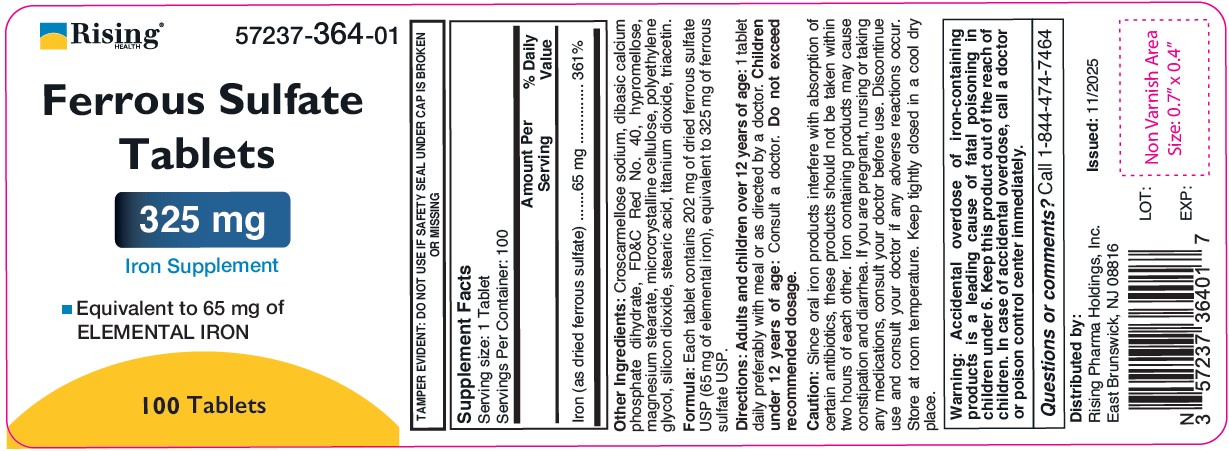
Supplement Facts
Serving size: 1 Tablet
Servings Per Container: 100 and 1,000
Amount Per Serving % Daily Value Iron (as dried ferrous sulfate) 65 mg 361% Other Ingredients: Croscarmellose sodium, dibasic calcium phosphate dihydrate, FD&C Red No. 40, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, silicon dioxide, stearic acid, titanium dioxide, triacetin.
Formula: Each tablet contains 202 mg of dried ferrous sulfate USP (65 mg of elemental iron), equivalent to 325 mg of ferrous sulfate USP.
- Directions:
-
Caution:
Since oral iron products interfere with absorption of certain antibiotics, these products should not be taken within two hours of each other. Iron containing products may cause constipation and diarrhea. If you are pregnant, nursing or taking any medications, consult your doctor before use. Discontinue use and consult your doctor if any adverse reactions occur.
Store at room temperature. Keep tightly closed in a cool dry place.
Warning: Accidental overdose of iron-containing products is a leading cause of fatal poisoning in children under 6. Keep this product out of the reach of children. In case of accidental overdose, call a doctor or Poison Control Center immediately. - SAFE HANDLING WARNING
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
FERROUS SULFATE
ferrous sulfate tablet, film coatedProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:57237-364 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FERROUS SULFATE (UNII: 39R4TAN1VT) (FERROUS CATION - UNII:GW89581OWR) FERROUS CATION 65 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) DIBASIC CALCIUM PHOSPHATE DIHYDRATE (UNII: O7TSZ97GEP) FD&C RED NO. 40 (UNII: WZB9127XOA) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) CELLULOSE, MICROCRYSTALLINE (UNII: OP1R32D61U) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) STEARIC ACID (UNII: 4ELV7Z65AP) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) TRIACETIN (UNII: XHX3C3X673) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:57237-364-01 100 in 1 BOTTLE, PLASTIC 2 NHRIC:57237-364-10 1000 in 1 BOTTLE, PLASTIC Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date dietary supplement 12/22/2025 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color shape scoring 1 size (solid drugs) 10 mm Labeler - Rising Pharma Holdings, Inc. (116880195) Registrant - Nucrogene Inc (110928621) Establishment Name Address ID/FEI Business Operations Nucrogene Inc 110928621
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.