EXZOLT CATTLE-CA1- fluralaner solution
EXZOLT CATTLE-CA1 by
Drug Labeling and Warnings
EXZOLT CATTLE-CA1 by is a Animal medication manufactured, distributed, or labeled by Merck Sharp & Dohme Corp.. Drug facts, warnings, and ingredients follow.
Drug Details [pdf]
- SPL UNCLASSIFIED SECTION
- Caution:
-
Description:
Exzolt Cattle-CA1 (fluralaner topical solution) contains fluralaner, an antiparasitic of the isoxazoline class. Each mL of Exzolt Cattle-CA1 contains 50 mg of fluralaner.
The chemical name of fluralaner is (±)-4-[5-(3,5-dichlorophenyl)-5-(trifluoromethyl)-4,5-dihydroisoxazol-3-yl]-2-methyl-N-[2-oxo-2-(2,2,2-trifluoroethylamino)ethyl]benzamide.
Inactive Ingredients: pyrrolidone, isopropyl alcohol, l-menthol, propylene glycol dicaprylate/dicaprate, FD&C blue No. 1, FD&C yellow No. 5
-
Indications for Use:
Exzolt Cattle-CA1 is indicated for the prevention and treatment of infestations caused by New World screwworm (Cochliomyia hominivorax) larvae (myiasis) and treatment and control of cattle fever tick (Rhipicephalus microplus) in beef cattle 2 months of age and older and replacement dairy heifers less than 20 months of age. Not for use in bulls intended for breeding 1 year of age and older, dairy calves, and veal calves.
-
Dosage and Administration:
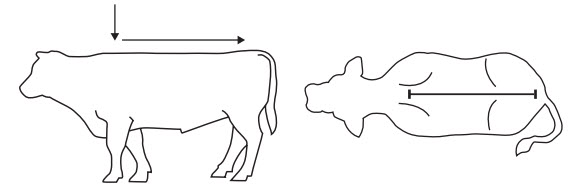
Exzolt Cattle-CA1 is a ready-to-use topical formulation intended for direct application to the hair and skin in a narrow strip extending along the dorsal midline from the withers to the base of the tail (see Figure 1). The recommended rate of administration is 1 mL/44.1 lbs. (1 mL/20 kg) body weight, which is equivalent to 1.13 mg of fluralaner for each pound (2.5 mg/kg) body weight. Effectiveness has not been evaluated in cattle with wet hides.
Recommended site of administration:
Figure 1: Recommended location for the topical application in a narrow strip along the dorsal midline from the withers to the base of the tail.
Administration of the product with 250 mL and 1L bottles with built-in dosing chamber:
To ensure administration of a correct dose, body weight should be determined as accurately as possible, and accuracy of the dosing volume should be checked before administration. Round the dose up to the nearest volume increment on the dosing chamber, which goes up in 2.5 mL increments.
The table below can be consulted to assist in the calculation of the appropriate volume which must be applied based on the weight of animal being treated.
Body Weight (Pounds) Dose Volume (mL) - * Add 2.5 mL for each 110 pounds above 1320 pounds of body weight.
220 5 330 7.5 440 10 550 12.5 660 15 770 17.5 880 20 990 22.5 1100 25 1320* 30 Practice the Administration and Overfill Reduction Instructions a few times to become familiar with operating the package before dosing animals.
Step 1
On first use
remove cap and peelable seal from the dosing chamber.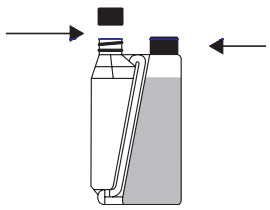
Do not remove cap from the bottle.Step 2
Dosing chamber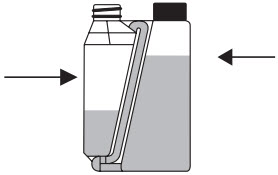
Hold the bottle upright and at eye level while slowly and gently squeezing the bottle to fill the dosing chamber to the selected mark. Step 3 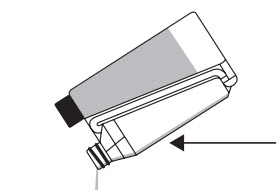
Pour the measured volume on the dorsal midline from withers to the base of the tail. Application to a small area should be avoided. A small amount of liquid will remain on the walls of the chamber, but the chamber is calibrated to account for this.
Avoid squeezing the container section while the solution is poured from the dosing chamber.
If the dosing chamber is overfilled follow the Overfill Reduction Instructions below:
Step 1
Re-apply cap to dosing chamber and tighten.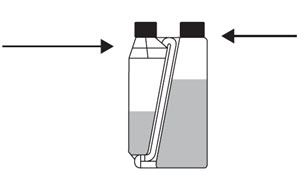
Confirm the cap is tight.Step 2
Transfer Tube Air Pocket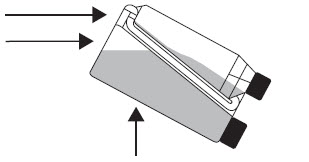
Tilt the bottle to allow an air pocket to form at the beginning of the transfer tube inside the bottle. Step 3 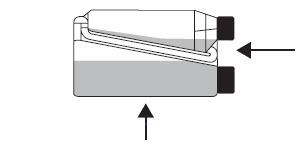
Transfer Tube Hold the bottle horizontally to allow product to cover the end of the transfer tube inside the dosing chamber. Step 4 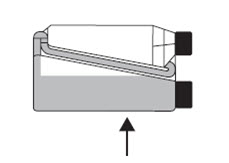
Squeeze and release the bottle repeatedly.
Product will return to the bottle through the transfer tube.
Administration of the product with 5L bottle with an applicator device:
These bottles are designed for use with the Simcro Breaze™ Applicator Device (30 mL). This applicator device and delivery tubing (sold separately by Simcro as a kit) should be used with the 5L bottle. The 5L bottle is supplied with spigot cap attached to dip tube for its use with the applicator device. A strap is also included for use of the 5L bottle as a backpack.
To ensure administration of a correct dose, body weight should be determined as accurately as possible, and accuracy of the dosing volume should be checked before administration. Round the dose up to the nearest volume increment on the applicator device, which goes up in 1 mL increments.
The table below can be consulted to assist in the calculation of the appropriate volume which must be applied based on the weight of animal being treated.
Assembly, Disassembly and Cleaning Instructions for the 5L bottle with applicator device:
Step 1
Follow the applicator device manufacturer's assembly directions. Connect one end of the delivery tubing to the connection point on the dosing applicator.
Step 2
Remove the transit cap and protection seal from the 5L bottle and replace with spigot cap attached to dip tube. Tighten spigot cap to bottle and attach other end of delivery tubing to the spigot cap. Do not discard the transit cap until the contents of the 5L bottle are completely used. Please refer to Figure 2 for the assembled 5L bottle with applicator device.
Step 3
Keeping the 5L bottle in an upright position, gently prime the applicator device per the included manufacturer's instructions, checking for leaks. With the applicator device in an upward position, expel all visible air from the barrel and confirm that product is visibly expressed from the tip of the applicator device so that it is free of any residual air.
Step 4
Follow the applicator device manufacturer's directions for adjusting the dose.
Step 5
When the interval between uses of the applicator device is expected to exceed 1 week, take off the entire spigot assembly (delivery tubing connected to the spigot cap with attached dip tube while still connected to the applicator device), from the 5L bottle. Return any unused product remaining in the applicator device and in the delivery tubing back into the 5L bottle. Raise the spigot cap with dip tube attached and place the tip of the applicator device into the 5L bottle. Discharge the remaining product from the spigot assembly into the bottle. Place the transit cap onto the 5L bottle to close it. Submerge the dip tube in warm, soapy water. Flush warm soapy water through the delivery tubing and through the applicator device, followed by flushing them with clean water and allowing them to dry. Once dry, store the entire dosing assembly (applicator device, delivery tubing, spigot cap with attached dip tube) in a safe, clean place until next use. Refer to the manufacturer's directions for maintenance of the applicator.
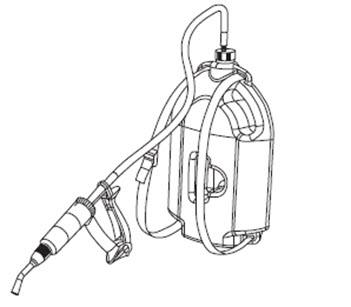
Figure 2. 5L bottle and applicator device with tubing.
-
Warnings:
WITHDRAWAL PERIODS AND RESIDUE WARNINGS:
Environmental temperature affects the withdrawal period.
Cattle must not be slaughtered for human consumption within 98 days of treatment.
If cattle are continuously exposed to temperatures at or above 60° F after product administration, then cattle may be slaughtered for human consumption 44 days after treatment. Violative residues may result if cattle are exposed to temperatures below 60° F after administration and are slaughtered at 44 days.
Not for use in female dairy cattle 20 months of age or older, including dry dairy cows; use in these cattle may cause drug residues in milk and/or in calves born to these cows or heifers. Not for use in beef calves less than 2 months of age, dairy calves, and veal calves. A withdrawal period has not been established for this product in pre-ruminating calves.
User Safety Warnings:
Not for use in humans. Keep out of reach of children.
This drug product is a skin and eye irritant; special care should be taken to avoid contact. Personal protective equipment should be worn, such as gloves, long sleeve shirt and pants, as well as glasses or goggles to prevent skin, eye and mucous membrane contact and/or drug absorption, while handling the product. In case of skin contact, wash with soap and water. If contact with eyes occurs, immediately rinse thoroughly with water. In case of accidental spill, immediately remove affected clothing and wash contacted skin with soap and water. In case of accidental ingestion, immediately rinse the mouth with plenty of water and seek medical advice.
Do not eat, drink, or smoke while handling the product. Wash hands thoroughly with soap and water immediately after use of the product.
The product is highly flammable. Keep away from heat, sparks, open flame or other sources of ignition.
To obtain a Safety Data Sheet (SDS) or for technical assistance, call Merck Animal Health at 1-800-211-3573.
Contact Information:
Contact Merck Animal Health at 1-800-211-3573 or https://www.merck-animal-health-usa.com.
To report suspected adverse drug experiences, contact Livestock Technical Service at 1-800-211-3573. For additional information about reporting adverse drug experiences for animal drugs, contact FDA at 1-888-FDA-VETS or https://www.fda.gov/reportanimalae.
-
CLINICAL PHARMACOLOGY
Clinical Pharmacology:
Mechanism of Action
Fluralaner belongs to the class of isoxazoline-substituted benzamide derivatives. Fluralaner is an inhibitor of the arthropod nervous system. The mode of action of fluralaner is the antagonism of the ligand-gated chloride channels (gamma-aminobutyric acid (GABA)-receptor and glutamate-receptor).
Pharmacokinetics
The pharmacokinetic properties of a single 2.5 mg/kg dose of Exzolt Cattle-CA1 administered topically along the dorsal midline from the withers to the base of the tail to cattle that were not restricted from grooming are presented in Table 1 (n = 12).
Table 1: Mean (± standard deviation) plasma pharmacokinetic parameters of total fluralaner* after a single topical administration of Exzolt Cattle-CA1 in male and female cattle in warm conditions (54 – 98 °F) Parameter (units) Estimate Cmax = maximum plasma concentration Tmax = time to maximum plasma concentration AUC(0-56) represents the AUC from day 0 to day 56 AUCinf = area under the curve from the time of dosing extrapolated to infinity t½ = half-life - * Although total fluralaner (R+S) is reported, the S enantiomer is more abundant and active than the R
- † Median and range
Cmax (ng/mL) 127 ± 82.2 Tmax† (day) 5 (4 – 12) AUC(0-56) (day*ng/mL) 1570 ± 1220 AUCinf (day*ng/mL) 1590 ± 1230 t½ (day) 8.48 ± 1.84 -
SPL UNCLASSIFIED SECTION
Target Animal Safety
Margin of Safety:
In a margin of safety study, Exzolt Cattle-CA1 was well tolerated in 32 six to seven month old healthy beef cattle (16 males and 16 females). Study animals were administered 3.7, 11.1, or 18.5 mg/kg body weight (1X, 3X, and 5X the maximum anticipated labeled dose) of Exzolt Cattle-CA1 by topical application three times 42 days apart (Days 0, 42, and 84). Cattle in the control group (0X) were treated with green dyed sterile saline at a dose volume similar to the 5X treated group. General health observations were conducted twice daily from acclimation to the end of the 98-day study. Variables measured periodically throughout the study for each animal were body weight; physical examinations; neurological examinations; analysis of blood for hematology, clinical chemistry, coagulation, and toxicokinetics; fecal and urine analysis; and feed and water consumption. All animals were necropsied at the end of the study for gross and histopathological examination and select organs were weighed.
Test article-related application site reactions, including skin flaking/scurfing and scabbing were observed. These findings were dose-dependent in both incidence and severity. Reactions in the 1X animals appeared after the second administration. These reactions in the 1X group were cosmetic in nature and did not require treatment.
Female Reproductive Safety:
In a reproductive safety study, Exzolt Cattle-CA1 was well tolerated in 200 healthy beef cows between the ages of 3 to 11 years old. Study animals were administered 11.1 mg fluralaner/kg body weight (3X the maximum labeled dose) of Exzolt Cattle-CA1 by a single topical application once during breeding (estrus; before timed-artificial insemination), early in the 1st trimester of pregnancy, during the mid-1st trimester of pregnancy, or in the 3rd trimester of pregnancy. Cattle in the control group (0X) were treated with green dyed sterile saline at a dose volume similar to the treated groups (3X). General health observations were conducted twice daily from acclimation to the end of the study at 30±2 days postpartum. Variables measured at start of acclimation and at the end of the study for each animal were body weight (including prior to each dosing) and physical examinations (including at parturition for offspring). Reproductive safety parameters included conception rate, abortion rate, calving rate, live births, stillborn calves, perinatal death, premature deliveries, neonatal death, dystocia, ability of calf to stand, walk and suckle, and abnormalities. Three stillbirths and one premature delivery were observed in animals in the control group. One stillbirth associated with dystocia and one premature delivery were documented in cows treated with Exzolt Cattle-CA1.
Six abortions occurred across three of the Exzolt Cattle-CA1 treated groups (2 out of 31 cows in the estrus-treated group; 2 out of 34 cows in the early first trimester-treated group; 2 out of 27 cows in the mid first trimester-treated group). One calf was found dead within 24 hours of birth in an Exzolt Cattle-CA1 treated cow. These events were considered to occur at rates typical for the source herd and unlikely to be test article related. Not for use in bulls intended for breeding over 1 year of age, as reproductive safety has not been evaluated.
-
SPL UNCLASSIFIED SECTION
Reasonable Expectation of Effectiveness
A reasonable expectation of effectiveness may be demonstrated based on evidence such as, but not limited to, pilot data in the target species or studies from published literature.
Exzolt Cattle-CA1 is conditionally approved pending a full demonstration of effectiveness. Additional information for Conditional Approvals can be found at www.fda.gov/animalca. A reasonable expectation of effectiveness for Exzolt Cattle-CA1 for the prevention and treatment of infestations caused by New World screwworm (Cochliomyia hominivorax) larvae (myiasis) and treatment and control of cattle fever tick (Rhipicephalus microplus) in beef cattle 2 months of age and older and replacement dairy heifers less than 20 months of age is based on results from the following foreign studies conducted in Australia, Brazil, and South Africa.
- A.
New World Screwworm (NWS) (Cochliomyia hominivorax) Three effectiveness studies utilizing natural NWS infestations conducted in Brazil in 2018 are described below:
- Support for a prevention indication: This study evaluated prevention of New World Screwworm (NWS) myiasis in a surgical wound created seven days after treatment administration. Animals received either a placebo (n=6) or Exzolt Cattle-CA1 (n=6) on Day -7. Seven days later, two surgical incisions were made on each side of the body at the shoulder. Animals were housed outside to facilitate natural infestation of the wounds with NWS. Cattle were monitored twice daily for 10 days post-incision to assess the presence of eggs and larvae. A single topical application of Exzolt Cattle-CA1 at the dose of 2.5 mg/kg provided 100% prevention against myiasis for the length of the study.
- Support for a prevention indication: This study evaluated prevention of NWS myiasis in a castration wound created on the day of treatment with either a placebo (n=15) or Exzolt Cattle-CA1 (n=15). Animals were housed outside to facilitate natural infestation of the wounds with NWS. Cattle were monitored daily for 14 days post-surgery to assess the presence of eggs, larvae, and the progress of wound healing. A single topical administration of Exzolt Cattle-CA1 at the dose of 2.5 mg/kg provided 100% prevention against myiasis for up to 14 days following castration.
- Support for a therapeutic indication: This study evaluated the effectiveness of the product to treat a wound already infested with NWS. A surgical wound was created and left exposed to facilitate natural infestation with NWS. Three days later, after confirming the presence of live larvae, animals were treated topically once with either a placebo (n=12) or Exzolt Cattle-CA1 (n=12). A single topical administration of Exzolt Cattle-CA1 at the dose of 2.5 mg/kg achieved 90.9% effectiveness by the second day post-treatment and reached 100% effectiveness by the third day. No myiasis in treated animals was observed up to day 5.
- B. Cattle Fever Tick (Rhipicephalus microplus)
Three dose confirmation studies conducted in Brazil and South Africa and a rain exposure study conducted in Brazil utilizing induced infestations of R. microplus were evaluated. These studies were conducted between 2018 and 2021. In each study, animals were individually housed and randomly assigned to control and Exzolt Cattle-CA1-treated groups. Exzolt Cattle-CA1-treated groups received a single administration at the dose of 2.5 mg/kg. A total of thirty animals were treated with Exzolt Cattle-CA1 across these four studies. The product demonstrated 100% effectiveness within the first week after Exzolt Cattle-CA1 administration. Length of consistent 100% persistent effectiveness ranged from 39 days to approximately 110 days post-treatment.
Thirteen field effectiveness studies conducted in Brazil and Australia utilizing natural infestations of R. microplus were evaluated. These studies were conducted between 2017 and 2023. In each study, animals were grouped housed and randomly assigned to control and Exzolt Cattle-CA1-treated groups. Exzolt Cattle-CA1-treated groups received a single administration at the dose of 2.5 mg/kg. Approximately 220 animals were treated with Exzolt Cattle-CA1 across these thirteen studies. The product demonstrated 100% effectiveness within the first week after Exzolt Cattle-CA1 administration. Length of consistent 100% persistent effectiveness ranged from 28 days to 70 days post-treatment.- C. Rain exposure study
One study was conducted to evaluate the impact of simulated rainfall post-treatment on the effectiveness of Exzolt Cattle-CA1 with cattle artificially infested with R. microplus. A total of 30 cattle (cross-bred beef bulls) were randomized to one of five groups with six animals each: Groups A, B, C, and D were treated with Exzolt Cattle-CA1 (2.5 mg/kg) and Group E with saline (equivalent volume). Groups A, B, and C were exposed to simulated rainfall at the following post-treatment timepoints: 6 hr, 12 hr, and 24 hr, respectively. Groups D and E had no exposure to rain. Percent effectiveness of Exzolt Cattle-CA1 was 100% in Groups A, B, C, and D up to 77 days. Rain exposure as early as 6 hr post-treatment did not affect the therapeutic or persistent effectiveness of Exzolt Cattle-CA1 in beef cattle.
- A.
New World Screwworm (NWS) (Cochliomyia hominivorax) Three effectiveness studies utilizing natural NWS infestations conducted in Brazil in 2018 are described below:
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 5L Bottle Carton
Exzolt™ Cattle-CA1
(fluralaner topical solution)Antiparasitic
50 mg of fluralaner/mL
Caution:
Federal law restricts this drug to use by or on the
order of a licensed veterinarian.Conditionally approved by FDA pending a full
demonstration of effectiveness under application
number 141-617.It is a violation of Federal law to use this product other than
as directed in the labeling.Indications for Use:
Exzolt Cattle-CA1 is indicated for the prevention and
treatment of infestations caused by New World screwworm
(Cochliomyia hominivorax) larvae (myiasis) and treatment
and control of cattle fever tick (Rhipicephalus microplus) in
beef cattle 2 months of age and older and replacement
dairy heifers less than 20 months of age. Not for use in
bulls intended for breeding 1 year of age and older, dairy
calves, and veal calves.Net Contents: 5L
MERCK
Animal Health
-
INGREDIENTS AND APPEARANCE
EXZOLT CATTLE-CA1
fluralaner solutionProduct Information Product Type PRESCRIPTION ANIMAL DRUG Item Code (Source) NDC: 0061-1442 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength FLURALANER (UNII: WSH8393RM5) (FLURALANER - UNII:WSH8393RM5) FLURALANER 50 mg in 1 L Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL DICAPRYLATE (UNII: 581437HWX2) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 FREE ACID (UNII: 6TP696149N) ISOPROPYL ALCOHOL (UNII: ND2M416302) PYRROLIDONE (UNII: KKL5D39EOL) LEVOMENTHOL (UNII: BZ1R15MTK7) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC: 0061-1442-01 0.25 L in 1 BOTTLE, PLASTIC 2 NDC: 0061-1442-02 1 L in 1 BOTTLE, PLASTIC 3 NDC: 0061-1442-03 5 L in 1 BOTTLE, WITH APPLICATOR Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date Conditional NADA NADA141617 12/05/2025 Labeler - Merck Sharp & Dohme Corp. (001317601)
© 2026 FDA.report
This site is not affiliated with or endorsed by the FDA.